Abstract
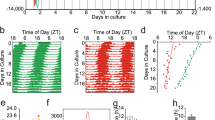
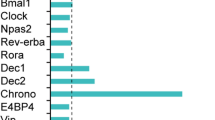
To examine for circadian rhythmicity, the messenger RNA (mRNA) amount of the clock genes Per1 and Per2 was measured in undifferentiated and nerve-growth-factor-differentiated PC12 cells harvested every fourth hour. Serum shock was needed to induce circadian oscillations, which in undifferentiated PC12 cultures lasted only one 24-h period, while in differentiated cultures, the rhythms continued for at least 3 days. Thus, neuronal differentiation provided PC12 cells the ability to maintain rhythmicity for an extended period. Both vasoactive intestinal polypeptide (VIP) and its receptor VPAC2 are expressed in the suprachiasmatic nucleus (SCN), and in agreement with VIP signaling being crucial for maintenance of rhythmicity, we found both VIP and VPAC2 mRNA increased after differentiation of PC12 cells. Pituitary adenylate cyclase activating polypeptide (PACAP) exerts time- and concentration-dependent effects on Per gene expression in the SCN. We added 1 nM and 1 μM PACAP to oscillating PC12 cells at times corresponding to midday and early and late night to evaluate whether the effects were similar as in SCN. Induction of Per1 mRNA was found at all three times, which differs from results in SCN. Thus, PC12 cells seem more useful for studying mechanisms behind acquirement of rhythmicity of cell cultures than for resetting of circadian rhythm.




Similar content being viewed by others
Abbreviations
- HS:
-
horse serum
- NGF:
-
nerve growth factor
- PACAP:
-
pituitary adenylate cyclase activating polypeptide
- PBS:
-
phosphate-buffered saline
- RHT:
-
retinohypothalamic tract
- RT-PCR:
-
reverse transcription polymerase chain reaction
- SCN:
-
suprachiasmatic nucleus
- VIP:
-
vasoactive intestinal polypeptide
- ZT:
-
zeitgeber time
References
Abrahamson, E. E., & Moore, R. Y. (2001). Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Research, 916, 172–191.
Akashi, M., & Nishida, E. (2000). Involvment of the MAP kinase cascade in resetting of the mammalian circadian clock. Genes & Development, 14, 645–649.
Albrecht, U., Sun, Z. S., Eichele, G., & Lee, C. C. (1997). A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell, 91, 1055–1064.
Allen, G., Rappe, J., Earnest, D. J., & Cassone, V. M. (2001). Oscillating on borrowed time: Diffusible signals from immortalized suprachiasmatic nucleus cells regulate circadian rhythmicity in cultured fibroblasts. Journal of Neuroscience, 21, 7937–7943.
Aton, S. J., Colwell, C. S., Harmar, A. J., Waschek, J., & Herzog, E. D. (2005). Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nature Neuroscience, 8, 476–483.
Balsalobre, A., Brown, S. A., Marcacci, L., Tronche, F., Kellendonk, C., Reichardt, H. M., et al. (2000a). Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science, 289, 2344–2347.
Balsalobre, A., Damiola, F., & Schibler, U. (1998). A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell, 93, 929–937.
Balsalobre, A., Marcacci, L., & Schibler, U. (2000b). Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Current Biology, 10, 1291–1294.
Berson, D. M. (2003). Strange vision: ganglion cells as circadian photoreceptors. Trends in Neuroscience, 26, 314–320.
Brown, S. A., Zumbrunn, G., Fleury-Olela, F., Preitner, N., & Schibler, U. (2002). Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Current Biology, 12, 1574–1583.
Chomczynski, P., & Sacchi, N. (1987). Single-step methods of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry, 162, 156–159.
Colwell, C. S., Michel, S., Itri, J., Rodriguez, W., Tam, J., Lelievre, V., et al. (2003). Disrupted circadian rhythms in VIP and PHI-deficient mice. American Journal of Physiology—Regulatory Integrative and Comparative Physiology, 285, R939–R949.
Cutler, D. J., Haraura, M., Reed, H. E., Shen, S., Sheward, W. J., Morrison, C. F., et al. (2003). The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. European Journal of Neuroscience, 17, 197–204.
Fahrenkrug, J. (2006). PACAP—A multifacetted neuropeptide. Chronobiology International, 23, 53–61.
Fahrenkrug, J., Georg, B., Hannibal, J., Hindersson, P., & Gräs, S. (2006). Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology, 147, 3769–3776.
Fahrenkrug, J., & Hannibal, J. (1998). Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience, 83, 1261–1272.
Fujioka, A., Takashima, N., & Shigeyoshi, Y. (2006). Circadian rhythm generation in a glioma cell line. Biochemical and Biophysical Research Communications, 346, 169–174.
Georg, B., Wulff, B. S., & Fahrenkrug, J. (1994). Characterization of the effects of retinoic acid on vasoactive intestinal polypeptide gene expression in neuroblastoma cells. Endocrinology, 135, 1455–1463.
Greene, L. A., Farinelli, S. E., Cunningham, M. E., & Park, D. S. (1998). Culture and experimental use of the PC12 rat pheochromocytoma cell line. In G. Banker, G. K Goslin (Eds.), Culturing nerve cells, 2nd edn (pp. 161–188). London: Bradford.
Greene, L. A., & Tischler, A. S. (1976). Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proceedings of the National Academy of Sciences USA, 73, 2424–2428.
Hannibal, J. (2002). Pituitary adenylate cyclase-activating peptide in the rat central nervous system: An immunohistochemical and in situ hybridization study. Journal of Comparative Neurology, 453, 389–417.
Hannibal, J. (2006). Roles of PACAP-containing retinal ganglion cells in circadian timing. International Review of Cytology, 251, 1–41.
Hannibal, J., Moller, M., Ottersen, O. P., & Fahrenkrug, J. (2000). PACAP and glutamate are co-stored in the retinohypothalamic tract. Journal of Comparative Neurology, 418, 147–155.
Harmar, A. J., Marston, H. M., Shen, S., Spratt, C., West, K. M., Sheward, W. J., et al. (2002). The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell, 109, 497–508.
Hirota, T., Okano, T., Kokame, K., Shirotani-Ikejima, H., Miyata, T., & Fukada, Y. (2002). Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured rat-1 fibroblasts. Journal of Biological Chemistry, 277, 44244–44251.
Honma, S., Katsuno, Y., Tanahashi, Y., Abe, H., & Honma, K. (1998). Circadian rhythms of arginine vasopressin and vasoactive intestinal polypeptide do not depend on cytoarchitecture of dispersed. Neuroscience, 86, 967–976.
Kaeffer, B., & Pardini, L. (2005). Clock genes of mammalian cells: practical implications in tissue culture. In Vitro Cellular & Developmental Biology—Animal, 41, 311–320.
Lowrey, P. L., & Takahashi, J. S. (2004). Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annual Review of Genomics and Human Genetics, 5, 407–441.
Matsushita, T., Amagai, Y., Terai, K., Kojima, T., Obinata, M., & Hashimoto, S. (2006). A novel neuronal cell line derived from the ventrolateral region of the suprachiasmatic nucleus. Neuroscience, 140, 849–856.
Maywood, E. S., Reddy, A. B., Wong, G. K. Y., O’Neill, J. S., O’Brien, J. A., McMahon, D. G., et al. (2006). Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Current Biology, 16, 599–605.
Menger, G. J., Lu, K., Thomas, T., Cassone, V. M., & Earnest, D. J. (2005). Circadian profiling of the transcriptome in immortalized rat SCN cells. Physiological Genomics, 21, 370–381.
Minami, Y., Furuno, K., Akiyama, M., Moriya, T., & Shibata, S. (2002). Pituitary adenylate cyclase-activating polypeptide produces a phase shift associated with induction of mPer expression in the mouse suprachiasmatic nucleus. Neuroscience, 113, 37–45.
Moore, R. Y., Speh, J. C., & Leak, R. K. (2002). Suprachiasmatic nucleus organization. Cell and Tissue Research, 309, 89–98.
Nagoshi, E., Saini, C., Bauer, C., Laroche, T., Naef, F., & Schibler, U. (2004). Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell, 119, 693–705.
Nelson, W., Tong, Y. L., Lee, J. K., & Halberg, F. (1979). Methods for cosinor-thythmometry. Chronobiologia, 6, 305–323.
Nielsen, H. S., Hannibal, J., Knudsen, S. M., & Fahrenkrug, J. (2001). Pituitary adenylate cyclase activating polypeptide induces period 1 and period 2 gene expression in the rat suprachiasmatic nucleus during late night. Neuroscience, 103, 433–441.
Prolo, L. M., Takahashi, J. S., & Herzog, E. D. (2005). Circadian rhythm generation and entrainment in astrocytes. Journal of Neuroscience, 25, 404–408.
Reppert, S. M., & Weaver, D. R. (2002). Coordination of circadian timing in mammals. Nature, 418, 935–941.
Sanggaard, K. M., Hannibal, J., & Fahrenkrug, J. (2003). Serotonin inhibits glutamate - but not PACAP-induced per gene expression in the rat suprachiasmatic nucleus at night. European Journal of Neuroscience, 17, 1245–1252.
Shafer, T. J., & Atchison, W. D. (1991). Methylmercury blocks N- and L-type Ca++ channels in nerve growth factor-differentiated pheochromocytoma (PC12) cells. Journal of Pharmacology and Experimental Therapeutics, 258, 149–157.
Shearman, L. P., Zylka, M. J., Weaver, D. R., Kolakowski Jr., L. F., & Reppert, S. M. (1997). Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron, 19, 1261–1269.
Shigeyoshi, Y., Taguchi, K., Yamamoto, S., Takekida, S., Yan, L., Tei, H., et al. (1997). Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell, 91, 1043–1053.
Stratmann, M., & Schibler, U. (2006). Properties, entrainment, and physiological functions of mammalian peripheral oscillators. Journal of Biological Rhythms, 21, 494–506.
Sun, Z. S., Albrecht, U., Zhuchenko, O., Bailey, J., Eichele, G., & Lee, C. C. (1997). RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell, 90, 1003–1011.
Tei, H., Okamura, H., Shigeyoshi, Y., Fukuhara, C., Ozawa, R., Hirose, M., et al. (1997). Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature, 389, 512–516.
Welsh, D. K., Yoo, S. -H., Liu, A. C., Takahashi, J. S., & Kay, S. A. (2004). Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Current Biology, 14, 2289–2295.
Wulff, B. S., Georg, B., & Fahrenkrug, J. (1994). Expression and characterization of VIP and two VIP mutants in NIH 3T3 cells. FEBS Letters, 341, 43–48.
Yagita, K., & Okamura, H. (2000). Forskolin induces circadian gene expression of rPer1, rPer2 and dbp in mammalian rat-1 fibroblasts. FEBS Letters, 465, 79–82.
Yan, L., Takekida, S., Shigeyoshi, Y., & Okamura, H. (1999). Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience, 94, 141–150.
Zylka, M. J., Shearman, L. P., Weaver, D. R., & Reppert, S. M. (1998). Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron, 20, 1103–110.
Acknowledgments
The skilful technical assistance of Sanne S. Kelly, Lone K. Hellström, and Anita Hansen is gratefully acknowledged. Henrik L. Jørgensen, PhD is acknowledged for his help with the statistical analysis. The study was supported by The Danish Biotechnology Center for Cellular Communication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pretzmann, C.P., Fahrenkrug, J. & Georg, B. Differentiation of PC12 Cells Results in Enhanced VIP Expression and Prolonged Rhythmic Expression of Clock Genes. J Mol Neurosci 36, 132–140 (2008). https://doi.org/10.1007/s12031-008-9063-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-008-9063-9