Abstract
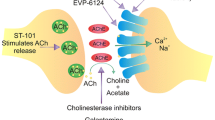
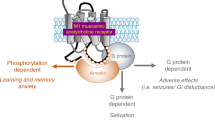
Loss of forebrain acetylcholine (ACh) is an early neurochemical lesion in Alzheimer’s Disease (AD), and muscarinic receptors for ACh are involved in memory and cognition, so a muscarinic agonist could provide ‘replacement therapy’ in this disease. Muscarinic receptors, which couple to G-proteins, occur throughout the CNS, and in the periphery they mediate the responses of the parasympathetic nervous system, so selectivity is crucial. The five subtypes of muscarinic receptor, M1–M5, have a distinct regional distribution, with M2 and M3 mediating most of the peripheral effects, M2 predominating in hindbrain areas, and M1 predominating in the cortex and hippocampus—the brain regions most associated with memory and cognition, which has lead to a search for a truly M1-selective muscarinic agonist. That search has so far been unsuccessful, but acetylcholinesterase inhibitors such as donepezil (Aricept), which potentiate cholinergic neurotransmission, have a therapeutic role in the management of AD; so the M1 receptor remains a therapeutic target. Our approach is to develop allosteric enhancers—compounds which bind to the receptor at an ‘allosteric’ site which is distinct from the ‘primary’ site to which the endogenous ligand binds, and which enhance the affinity (or efficacy) of the endogenous ligand. We have developed radioligand binding assays and analyses for the detection and quantitatitation of allosteric interactions of a test agent with labelled and unlabelled ‘primary’ ligands, and we report here some results of the initial phase of a chemical synthesis project to develop potent and selective allosteric enhancers at muscarinic M1 receptors.
Similar content being viewed by others
References
Birdsall N. J. M., Farries T., Gharagozloo P., Kobayashi S., Lazareno S., and Sugimoto M. (1999) Subtype-selective positive cooperative interactions between brucine analogs and acetylcholine at muscarinic receptors: functional studies. Mol. Pharmacol. 55, 778–786.
Caulfield M. and Straughan D. (1983) Muscarinic receptors revisited. Trends Neurisci. 6, 73–75.
Felder C. C., Bymaster F. P., Ward J., and DeLapp N. (2000) Therapeutic opportunities for muscarinic receptors in the central nervous system. J. Med. Chem. 43, 4333–4353.
Gharagozloo P., Lazareno S., Popham A., and Birdsall N. J. M. (1999) Allosteric interactions of quaternary strychnine and brucine derivatives with muscarinic acetylcholine receptors. J. Med. Chem. 42, 438–445.
Lazareno S. (1999) Measurement of agonist-stimulated [35S]GTP gamma S binding to cell membranes, in Receptor Binding Techniques, 106th ed. (Keen M., ed.), Humana Press, Totowa, NJ, pp. 231–245.
Lazareno S. and Birdsall N. J. M. (1993) Estimation of competitive antagonist affinity from functional inhibition curves using the Gaddum, Schild and Cheng-Prusoff Equations. Br. J. Pharmacol. 109, 1110–1119.
Lazareno S. and Birdsall N. J. M. (1995) Detection, quantitation, and verification of allosteric interactions of agents with labeled and unlabeled ligands at G protein-coupled receptors: interactions of strychnine and acetylcholine at muscarinic receptors. Mol. Pharmacol. 48, 362–378.
Lazareno S. and Birdsall N. J. M. (2000) Measurement of competitive and allosteric interactions in radioligand binding studies, in G Protein-Coupled Receptors (Haga T. and Berstein G., eds.), CRC Press, Boca Raton, pp. 1–48.
Lazareno S., Gharagozloo P., Kuonen D., Popham A., and Birdsall N. J. M. (1998) Subtype-selective positive cooperative interactions between brucine analogues and acetylcholine at muscarinic receptors: radioligand binding studies. Mol. Pharmacol. 53, 573–589.
Lazareno S., Popham A., and Birdsall N. J. M. (2000) Allosteric interactions of staurosporine and other indolocarbazoles with N-[methyl-(3)H]scopolamine and acetylcholine at muscarinic receptor subtypes: identification of a second allosteric site. Mol. Pharmacol. 58, 194–207.
Warburton D. M. (1975) Brain, Behaviour and Drugs. John Wiley & Sons, London.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lazareno, S., Popham, A. & Birdsall, N.J.M. Towards a high-affinity allosteric enhancer at muscarinic M1 receptors. J Mol Neurosci 19, 123–127 (2002). https://doi.org/10.1007/s12031-002-0022-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s12031-002-0022-6