Abstract
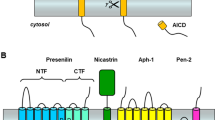
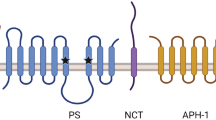
γ-Secretase processing of the amyloid-β precursor protein (APP) releases the amyloid-β peptide, which is widely held to be involved in the pathogenesis of Alzheimer’s disease. This protease is apparently a complex of integral membrane proteins that includes the multi-pass presenilin. Transition-state analogue inhibitors of γ-secretase are important molecular probes of the enzyme active site. We have identified new transition-state analogues, (hydroxyethy) urea peptidomimetics, that inhibit γ-secretase activity at submicromolar concentrations in cell culture. The inhibitory activity of a family of such compounds provided further support that γ-secretase has loose sequence specificity at the active site, and one of these compounds allowed partial purification of the protease complex. In addition, becuase the site of γ-secretase cleavage of APP lies within its single transmembrane domain, we designed short peptides based on this domain which assume a helical conformation. These peptides inhibited γ-secretase in the low micromolar range in cell culture, suggesting that they indeed mimick the APP substrate conformation.
Similar content being viewed by others
References
Cai H., Wang Y., McCarthy D., Wen H., Borchelt D. R., Price D. L., and Wong P. C. (2001) BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 4, 233–234.
Esler W. P., Kimberly W. T., Ostaszewski B. L., Diehl T. S., Moore C. L., Tsai J.-Y., et al. (2000) Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nature Cell Biol. 2, 428–434.
Esler W. P. and Wolfe M. S. (2001) A portrait of Alzheimer secretases: new features and familiar faces. Science 293, 1449–1454.
Esler W. P., Kimberly W. T., Ostaszewski B. L., et al. (2002) Activity-dependent isolation of the presenilin-γ-secretase complex reveals nicastrin and a γ substrate. Proc. Natl. Acad. Sci. USA 99, 2720–2725.
Flexner C. (1998) HIV-protease inhibitors. N. Engl. J. Med. 338, 1281–1292.
Getman D. P., DeCrescenzo G. A., Heintz R. M., Reed K. L., Talley J. J., Bryant M. L., et al. (1993) Discovery of a novel class of potent HIV-1 protease inhibitors containing the (R)-(hydroxyethyl)urea isostere. J. Med. Chem. 36, 288–291.
Greenlee W. J. (1990) Renin inhibitors. Med. Res. Rev. 10, 173–236.
Hong L., Koelsch G., Lin X., Wu S., Terzyan S., Ghosh A. K., Zhang X. C., and Tang J. (2000) Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science 290, 150–153.
Huff J. R. (1991) HIV protease: a novel chemotherapeutic target for AIDS. J. Med. Chem. 34, 2305–2314.
Judice J. K., Tom J. Y., Huang W., Wrin T., Vennari J., Petropoulos C. J., and McDowell R. S. (1997) Inhibition of HIV type 1 infectivity by constrained alpha-helical peptides: implications for the viral fusion mechanism. Proc. Natl. Acad. Sci. USA 94, 13426–13430.
Kimberly W. T., Xia W., Rahmati T., Wolfe M. S., and Selkoe D. J. (2000) The transmembrane aspartates in presenilin 1 and 2 are obligatory for γ-secretase activity and amyloid β-protein generation. J. Biol. Chem. 275, 3173–3178.
Li Y. M., Xu M-, Lai M-T., et al. (2000) Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin 1. Nature 405, 689–694.
Lichtenthaler S. F., Wang R., Grimm H., Uljon S. N., Masters C. L., and Beyreuther K. (1999) Mechanism of the cleavage specificity of Alzheimer’s disease gamma-secretase identified by phenylalanine-scanning mutagenesis of the transmembrane domain of the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 96, 3053–3058.
Luo Y., Bolon B., Kahn S., Bennett B. D., Babu-Khan S., Denis P., et al. (2001) Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 4, 231–232.
Moore C. L., Leatherwood D. D., Diehl T. S., Selkoe D. J., and Wolfe M. S. (2000) Difluoro ketone peptidomimetics suggest a large S1 pocket for Alzheimer’s γ-secretase: implications for inhibitor design. J. Med. Chem. 43, 3434–3442.
Thinakaran G., Harris C. L., Ratovitski T., Davenport F., Slunt H. H., Price D. L., et al. (1997) Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J. Biol. Chem. 272, 28415–28422.
Wolfe M. S. (2001) Secretase targets for Alzheimer’s disease: identification and therapeutic potential. J. Med. Chem. 44, 2039–2060.
Wolfe M. S., De Los Angeles J., Miller D. D., Xia W., and Selkoe D. J. (1999a) Are presenilins intramembrane-cleaving proteases? Implications for the molecular mechanism of Alzheimer’s disease. Biochemistry 38, 11223–11230.
Wolfe M. S., Esler W. P., Kimberly W. T., Diehl T. S., Rahmati T., and Selkoe. D. J. (2000) (Hydroxyethyl) urea-containing peptidomimetics: new molecular probes for γ-secretase/presenilin. Soc. Neurosci. 26, Abstr # 298.9.
Wolfe M. S. and Haass C. (2001) The role of presenilins in gamma-secretase activity. J. Biol. Chem. 276, 5413–5416.
Wolfe M. S., Xia W., Moore C. L., Leatherwood D. D., Ostaszewski B., Donkor I. O., and Selkoe D. J. (1999b) Peptidomimetic probes and molecular modeling suggest Alzheimer’s γ-secretases are intramembrane-cleaving aspartyl proteases. Biochemistry 38, 4720–4727.
Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., and Selkoe D. J. (1999c) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398, 513–517.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolfe, M.S., Esler, W.P. & Das, C. Continuing strategies for inhibiting alzheimer’s γ-secretase. J Mol Neurosci 19, 83–87 (2002). https://doi.org/10.1007/s12031-002-0015-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s12031-002-0015-5