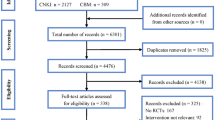
Abstract
Objective
Total neoadjuvant therapy (TNT) combining chemoradiotherapy (CRT) with chemotherapy (CT) was a novel pre-surgical approach to cancer treatment. This meta-analysis aimed to compare the clinical outcomes between neoadjuvant CRT (nCRT) with induction CT and nCRT with consolidated CT in locally advanced rectal cancer (LARC) patients.
Method
In July 2022, a literature search was conducted using the following public databases: PubMed, MEDLINE, Embase, the Cochrane Library, and Web of Science, retrieved all relevant articles comparing nCRT-combining induction CT with nCRT-combining-consolidated CT treatments for LARC patients.
Results
Four eligible studies were identified, including a total of 995 LARC patients: 473 in the nCRT with consolidated CT group and 522 in the nCRT with induction CT group. The organ preservation (OP) rate of the nCRT with consolidated CT group was higher than that of the nCRT with induction CT group (RR [relative risk]: 1.53; 95% CI (confidence interval): 1.09–2.14). The pathological complete response (PCR, RR: 1.22; 95% CI 0.37–2.17), the 3-year disease-free survival (DFS, RR 1.02; 95% CI 0.71–1.46), the local recurrence (LR, RR 0.98; 95% CI 0.52–1.85), rates of R0 resection (RR 0.74; 95% CI 0.55–1.10), compliance (RR 0.52; 95% CI 0.12–2.26), and grade 3-–4 toxicities (RR 0.78; 95% CI 0.57–1.06) were all similar between the two groups.
Conclusion
In this meta-analysis of TNT regimens for rectal cancer, consolidative CT following nCRT was associated with similar PCR, 3-year DFS, LR, R0 resection, compliance, and grade 3–4 toxicities compared to induction CT prior to nCRT but a higher rate of organ preservation.




Similar content being viewed by others
Data Availability
All the data for this article can be found on PubMed, MEDLINE, Embase, the Cochrane Library, and Web of Science.
References
Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics. CA Cancer J Clin. 2020;70:145–64.
De Felice F, et al. Total neoadjuvant treatment in locally advanced rectal cancer, Transl Oncol. 2021.
Kong JC, et al. Ann Surg Oncol. Total neoadjuvant therapy in locally advanced rectal cancer: a systematic review and meta analysis of oncological and operative outcomes. 2021;28:7476–86.
Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241–8.
Gao YH, Lin JZ, An X, et al. Neoadjuvant sandwich treatment with oxaliplatin and capecitabine administered prior to, concurrently with, and following radiation therapy in locally advanced rectal cancer: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2014;90:1153–60.
Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4: e180071.
Ludmir EB, et al. Total neoadjuvant therapy for rectal cancer: an emerging option. Cancer. 2017;123:1497–506.
Gilshtein H, Ghuman A, Dawoud M, Yellinek S, Kent I, Sharp SP, et al. Total Neoadjuvant Treatment for Rectal Cancer: Preliminary Experience. Ann Surg. 2021;87:708–13.
Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12:513–9.
Chau I, Brown G, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24:668–74.
Fernandez-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo Cancer de Recto 3 study. J Clin Oncol. 2010;28:859–65.
Perez K, Safran H, Sikov W, et al. Complete neoadjuvant treatment of rectal cancer: the Brown University Oncology Group CONTRE study. Am J Clin Oncol. 2017;40:283–7.
Kim SY, Joo J, Kim TW, et al. A randomized phase 2 trial of consolidation chemotherapy after preoperative chemoradiation therapy versus chemoradiation therapy alone for locally advanced rectal cancer: KCSG CO 14–03. Int J Radiat Oncol Biol Phys. 2018;101:889–99.
Nogue M, Salud A, Vicente P, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist. 2011;16:614–20.
Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomised trial. Ann Oncol. 2015;26:1722–8.
Marechal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomised multicentric phase II study. Ann Oncol. 2012;23:1525–30.
Sclafani F, Brown G, Cunningham D, et al. PAN-EX: a pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann Oncol. 2016;27:1557–65.
Garcia-Aguilar J, Patil S, Gollub MJ. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol. 2022;2546–56.
Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29–42.
Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702–15.
Fokas E, Allga¨uer M, Polat B, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37:3712–3222.
Garant A, Kavan P, Martin AG, et al. Optimizing treatment sequencing of chemotherapy for patients with rectal cancer: the KIR randomized phase II trial. Radiother Oncol. 2021;155:237–45.
Marco MR, Zhou L, Patil S, et al. Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy improves survival in patients with locally advanced rectal cancer: final results of a multicenter phase II trial. Dis Colon Rectum. 2018;61:1146–55.
Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 535 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27:834–42.
Nilsson PJ, van Etten B, Hospers GA, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer--the RAPIDO trial. BMC Cancer 201313:279.
Golo D, But-Hadzic J, Anderluh F, Brecelj E, Edhemovic I, Jeromen A, Omejc M, Oblak I, Secerov-Ermenc A, Velenik V. Induction chemotherapy, chemoradiotherapy and consolidation chemotherapy in preoperative treatment of rectal cancer - long-term results of phase II OIGIT-01 Trial. Radiol Oncol. 2018;52(3):267–74.
van Zoggel DMGI, Bosman SJ, Kusters M, et al. Preliminary results of a cohort study of induction chemotherapy-based treatment for locally recurrent rectal cancer. Br J Surg. 2018;105(4):447–52.
Conroy T, Lamfichekh N, Etienne P-L, et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol. 2020;38(suppl 15):4007.
Author information
Authors and Affiliations
Contributions
Pengkhun Nov acquisition of data, analyzing, interpretation of data, and drafting the article; Kunpeng Du and Jiqiang Li designing, revising, and guiding the study. The authors read and approved.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent for publication
All the authors of the article agreed to be published in the journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nov, P., Du, K., Huang, Z. et al. A Meta-analysis of Total Neoadjuvant Therapies Combining Chemoradiotherapy with Induction or Consolidated Chemotherapy for Locally Advanced Rectal Cancer. J Gastrointest Canc 54, 693–702 (2023). https://doi.org/10.1007/s12029-022-00864-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00864-6