Abstract
Purpose
To investigate whether the early perfusion change in hepatocellular carcinoma (HCC) predicts the long-term therapeutic response to atezolizumab plus bevacizumab.
Methods
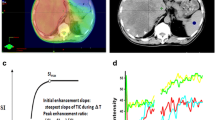
We retrospectively selected 19 subjects (median age: 62 years, 4 females, and 15 males) having advanced HCC and treated with atezolizumab alone (n = 3) or in combination with bevacizumab (n = 16). The 4-phased CT or MRI imaging was performed for each subject before and at 9 ± 2 and 21 ± 5 weeks after therapy initiation. The tumor-to-liver signal ratio in the arterial phase was used to estimate the tumor perfusion. The change in tumor perfusion from the baseline to the 1st follow-up exam was correlated with the tumor response evaluated using mRECIST at the 2nd follow-up exam. The difference between favorably responding and non-responding groups was statistically analyzed using one-way ANOVA.
Results
The mean tumor long axis in the baseline image was 59 ± 47 mm. The HCC perfusion changes were −26 ± 18% for complete (or partial) response (CR/PR, n = 8), −24 ± 12% for stable disease (SD, n = 8), and 9 ± 13% for progressive disease (PD, n = 3). The HCC perfusion change of the CR/PR groups was significantly lower than that of the PD group (p = 0.0040). The HCC perfusion changes between the SD and PD groups were also significantly different (p = 0.0135). The sensitivity and specificity of the early perfusion change to predict the long-term progression of the disease were 100 and 94%, respectively.
Conclusion
The early change in HCC perfusion may predict the long-term therapeutic response to atezolizumab plus bevacizumab, promoting personalized treatment for HCC patients.



Similar content being viewed by others
Data Availability
All data were collected from the UAB clinic database, which is not publicly available.
References
Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2-6.
Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–66.
Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–7.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905.
Yoo C, Kim JH, Ryu MH, et al. Clinical outcomes with multikinase inhibitors after progression on first-line atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: a multinational multicenter retrospective study. Liver Cancer. 2021;10(2):107–14.
Andrews A. Treating with checkpoint inhibitors-figure $1 million per patient. Am Health Drug Benefits. 2015;8(Spec Issue):9.
Verma V, Sprave T, Haque W, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer. 2018;6(1):128.
Wan X, Luo X, Tan C, Zeng X, Zhang Y, Peng L. First-line atezolizumab in addition to bevacizumab plus chemotherapy for metastatic, nonsquamous non-small cell lung cancer: a United States-based cost-effectiveness analysis. Cancer. 2019;125(20):3526–34.
Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11.
Liu X, Qin S. Immune checkpoint inhibitors in hepatocellular carcinoma: opportunities and challenges. Oncologist. 2019;24(Suppl 1):S3–10.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
Zheng X, Fang Z, Liu X, et al. Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J Clin Invest. 2018;128(5):2104–15.
Shigeta K, Datta M, Hato T, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 2020;71(4):1247–61.
Mpekris F, Voutouri C, Baish JW, et al. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc Natl Acad Sci U S A. 2020;117(7):3728–37.
Neter J, Kutner MH, Nachtsheim JC, Wasserman W. Applied linear statistical models. 4th ed. Columbus: The McGraw-Hill Companies Inc; 1996.
Rodgers JL, Nicewander WA. Thirteen ways to look at the correlation coefficient. Am Stat. 1988;42(1):59–66.
Fawcett T. An introduction to ROC analysis. Pattern Recogn Lett. 2006;27(8):861–74.
Heilmann M, Kiessling F, Enderlin M, Schad LR. Determination of pharmacokinetic parameters in DCE MRI: consequence of nonlinearity between contrast agent concentration and signal intensity. Invest Radiol. 2006;41(6):536–43.
Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues: I. literature survey. Phys Med Biol. 1996;41(11):2231–49.
Ingrisch M, Sourbron S. Tracer-kinetic modeling of dynamic contrast-enhanced MRI and CT: a primer. J Pharmacokinet Pharmacodyn. 2013;40(3):281–300.
Cao SE, Zhang LQ, Kuang SC, et al. Multiphase convolutional dense network for the classification of focal liver lesions on dynamic contrast-enhanced computed tomography. World J Gastroenterol. 2020;26(25):3660–72.
Kim H. Variability in quantitative DCE-MRI: sources and solutions. J Nat Sci. 2018;4(1).
Holland MD, Morales A, Simmons S, et al. Disposable point-of-care portable perfusion phantom for quantitative DCE-MRI. Med Phys. 2022;49(1):271–81.
Acknowledgements
The authors thank Mr. Morgan Amos, Ms. Amanda Richardson, Ms. Haley Hendrix, and Ms. Brandi Barger for collecting clinical data. This study was supported by the department of radiology incentive grant at UAB and the UAB comprehensive cancer center (grant P30 CA13148).
Funding
This work was supported by the department of radiology incentive grant at UAB and the UAB comprehensive cancer center (grant P30 CA13148).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Data collection and analysis were performed by Ezinwanne Onuoha and Andrew Smith. The first draft of the manuscript was written by Ezinwanne Onuoha, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki.
Consent to Participate
Informed consent was exempted for this retrospective study from UAB IRB.
Consent for Publication
Informed consent was exempted for this retrospective study from UAB IRB.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Onuoha, E., Smith, A.D., Cannon, R. et al. Perfusion Change of Hepatocellular Carcinoma During Atezolizumab plus Bevacizumab Treatment: A Pilot Study. J Gastrointest Canc 54, 776–781 (2023). https://doi.org/10.1007/s12029-022-00858-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00858-4