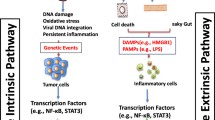
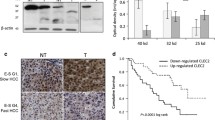
Abstract
Hepatocellular carcinoma is one of the major causes of cancer-related deaths worldwide and is associated with several inflammatory mediators, since 90% of HCCs occur based on chronic hepatitis B or C, alcoholism or increasingly metabolic syndrome-associated inflammation. EMT is a physiological process, with coordinated changes in epithelial gene signatures and is regulated by multiple factors, including cytokines and growth factors such as TGFβ, EGF, and FGF. Recent reports propose a strong association between EMT and inflammation, which is also correlated with tumor aggressiveness and poor outcomes. Cellular heterogeneity results collectively as an outcome of EMT, inflammation, and the tumor microenvironment, and it plays a fundamental role in the progression, complexity of cancer, and chemoresistance. In this review, we highlight recent developments concerning the association of EMT and inflammation in the context of HCC progression. Identifying potential EMT-related biomarkers and understanding EMT regulatory molecules will likely contribute to promising developments in clinical practice and will be a valuable tool for predicting metastasis in general and specifically in HCC.
Similar content being viewed by others
Data Availability
Not applicable.
References
Brabletz T. To differentiate or not–routes towards metastasis. Nat Rev Cancer. 2012;12(6):425–36. https://doi.org/10.1038/nrc3265.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. https://doi.org/10.1172/JCI39104.
Kim DH, Xing T, Yang Z, Dudek R, Lu Q, et al. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: a comprehensive overview. J Clin Med. 2017;7(1). https://doi.org/10.3390/jcm7010001.
Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol. 2012;366(1):34–54. https://doi.org/10.1016/j.ydbio.2011.12.041.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. https://doi.org/10.1016/j.cell.2009.11.007.
Yokoyama S, Asahara H. The myogenic transcriptional network. Cell Mol Life Sci. 2011;68(11):1843–9. https://doi.org/10.1007/s00018-011-0629-2.
Haensel D, Dai X. Epithelial-to-mesenchymal transition in cutaneous wound healing: where we are and where we are heading. Dev Dyn. 2018;247(3):473–80. https://doi.org/10.1002/dvdy.24561.
Stone RC, Pastar I, Ojeh N, Chen V, Liu S, et al. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016;365(3):495–506. https://doi.org/10.1007/s00441-016-2464-0.
Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020;30(10):764–76. https://doi.org/10.1016/j.tcb.2020.07.003.
Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell. 2019;49(3):361–74. https://doi.org/10.1016/j.devcel.2019.04.010.
Ribatti D, Tamma R, Annese T. Epithelial-mesenchymal transition in cancer: a historical overview. Transl Oncol. 2020;13(6): 100773. https://doi.org/10.1016/j.tranon.2020.100773.
Suarez-Carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017;11(7):805–23. https://doi.org/10.1002/1878-0261.12095.
Zhou C, Liu J, Tang Y, Liang X. Inflammation linking EMT and cancer stem cells. Oral Oncol. 2012;48(11):1068–75. https://doi.org/10.1016/j.oraloncology.2012.06.005.
Chan LK, Tsui YM, Ho DW, Ng IO. Cellular heterogeneity and plasticity in liver cancer. Semin Cancer Biol. 2021. https://doi.org/10.1016/j.semcancer.2021.02.015.
van Zijl F, Zulehner G, Petz M, Schneller D, Kornauth C, et al. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol. 2009;5(8):1169–79. https://doi.org/10.2217/fon.09.91.
Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401–35. https://doi.org/10.1007/978-3-0348-0837-8_16.
Refolo MG, Messa C, Guerra V, Carr BI, D'Alessandro R. Inflammatory mechanisms of HCC development. Cancers (Basel). 2020;12(3). https://doi.org/10.3390/cancers12030641.
Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2(1):6. https://doi.org/10.1038/s41698-018-0048-z.
Kew MC. Aflatoxins as a cause of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2013;22(3):305–10.
Severi T, van Malenstein H, Verslype C, van Pelt JF. Tumor initiation and progression in hepatocellular carcinoma: risk factors, classification, and therapeutic targets. Acta Pharmacol Sin. 2010;31(11):1409–20. https://doi.org/10.1038/aps.2010.142.
Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. 2006;80(6):1197–213. https://doi.org/10.1189/jlb.0506297.
Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20(23). https://doi.org/10.3390/ijms20236008.
Ding K, Fan L, Chen S, Wang Y, Yu H, et al. Overexpression of osteopontin promotes resistance to cisplatin treatment in HCC. Oncol Rep. 2015;34(6):3297–303. https://doi.org/10.3892/or.2015.4306.
Dong Q, Zhu X, Dai C, Zhang X, Gao X, et al. Osteopontin promotes epithelial-mesenchymal transition of hepatocellular carcinoma through regulating vimentin. Oncotarget. 2016;7(11):12997–3012. https://doi.org/10.18632/oncotarget.7016.
Cabiati M, Gaggini M, Cesare MM, Caselli C, De Simone P, et al. Osteopontin in hepatocellular carcinoma: a possible biomarker for diagnosis and follow-up. Cytokine. 2017;99:59–65. https://doi.org/10.1016/j.cyto.2017.07.004.
Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55(2):483–90. https://doi.org/10.1002/hep.24703.
Kim H, Park YN. Hepatocellular carcinomas expressing ‘stemness’-related markers: clinicopathological characteristics. Dig Dis. 2014;32(6):778–85. https://doi.org/10.1159/000368021.
Kong FF, Qu ZQ, Yuan HH, Wang JY, Zhao M, et al. Overexpression of FOXM1 is associated with EMT and is a predictor of poor prognosis in non-small cell lung cancer. Oncol Rep. 2014;31(6):2660–8. https://doi.org/10.3892/or.2014.3129.
Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, et al. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116(Pt 3):499–511.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96. https://doi.org/10.1038/nrm3758.
Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65(4):798–808. https://doi.org/10.1016/j.jhep.2016.05.007.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. https://doi.org/10.1038/nature01322.
Zhou YM, Cao L, Li B, Zhang RX, Sui CJ, et al. Clinicopathological significance of ZEB1 protein in patients with hepatocellular carcinoma. Ann Surg Oncol. 2012;19(5):1700–6. https://doi.org/10.1245/s10434-011-1772-6.
Sreekumar R, Emaduddin M, Al-Saihati H, Moutasim K, Chan J, et al. Protein kinase C inhibitors override ZEB1-induced chemoresistance in HCC. Cell Death Dis. 2019;10(10):703. https://doi.org/10.1038/s41419-019-1885-6.
Al-Ismaeel Q, Neal CP, Al-Mahmoodi H, Almutairi Z, Al-Shamarti I, et al. ZEB1 and IL-6/11-STAT3 signalling cooperate to define invasive potential of pancreatic cancer cells via differential regulation of the expression of S100 proteins. Br J Cancer. 2019;121(1):65–75. https://doi.org/10.1038/s41416-019-0483-9.
de Barrios O, Sanchez-Moral L, Cortes M, Ninfali C, Profitos-Peleja N, et al. ZEB1 promotes inflammation and progression towards inflammation-driven carcinoma through repression of the DNA repair glycosylase MPG in epithelial cells. Gut. 2019;68(12):2129–41. https://doi.org/10.1136/gutjnl-2018-317294.
Katsura A, Tamura Y, Hokari S, Harada M, Morikawa M, et al. ZEB1-regulated inflammatory phenotype in breast cancer cells. Mol Oncol. 2017;11(9):1241–62. https://doi.org/10.1002/1878-0261.12098.
Ricciardi M, Zanotto M, Malpeli G, Bassi G, Perbellini O, et al. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br J Cancer. 2015;112(6):1067–75. https://doi.org/10.1038/bjc.2015.29.
Gugnoni M, Ciarrocchi A. Long noncoding RNA and epithelial mesenchymal transition in cancer. Int J Mol Sci. 2019;20(8). https://doi.org/10.3390/ijms20081924.
Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1(6–7):303–14. https://doi.org/10.1002/emmm.200900043.
Aruga N, Kijima H, Masuda R, Onozawa H, Yoshizawa T, et al. Epithelial-mesenchymal transition (EMT) is correlated with patient’s prognosis of lung squamous cell carcinoma. Tokai J Exp Clin Med. 2018;43(1):5–13.
Deshmukh AP, Vasaikar SV, Tomczak K, Tripathi S, den Hollander P, et al. Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc Natl Acad Sci USA. 2021;118(19). https://doi.org/10.1073/pnas.2102050118.
Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7(344):re8. https://doi.org/10.1126/scisignal.2005189.
Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98(10):1512–20. https://doi.org/10.1111/j.1349-7006.2007.00550.x.
Fuxe J, Karlsson MC. TGF-beta-induced epithelial-mesenchymal transition: a link between cancer and inflammation. Semin Cancer Biol. 2012;22(5–6):455–61. https://doi.org/10.1016/j.semcancer.2012.05.004.
Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-beta - an excellent servant but a bad master. J Transl Med. 2012;10:183. https://doi.org/10.1186/1479-5876-10-183.
Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008;68(22):9107–11. https://doi.org/10.1158/0008-5472.CAN-08-2556.
Dituri F, Mancarella S, Cigliano A, Chieti A, Giannelli G. TGF-beta as multifaceted orchestrator in HCC progression: signaling, EMT, immune microenvironment, and novel therapeutic perspectives. Semin Liver Dis. 2019;39(1):53–69. https://doi.org/10.1055/s-0038-1676121.
Su Q, Fan M, Wang J, Ullah A, Ghauri MA, et al. Sanguinarine inhibits epithelial-mesenchymal transition via targeting HIF-1alpha/TGF-beta feed-forward loop in hepatocellular carcinoma. Cell Death Dis. 2019;10(12):939. https://doi.org/10.1038/s41419-019-2173-1.
Zhang J, Zhang Q, Lou Y, Fu Q, Chen Q, et al. Hypoxia-inducible factor-1alpha/interleukin-1beta signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology. 2018;67(5):1872–89. https://doi.org/10.1002/hep.29681.
Tam SY, Wu VWC, Law HKW. Hypoxia-induced epithelial-mesenchymal transition in cancers: HIF-1alpha and beyond. Front Oncol. 2020;10:486. https://doi.org/10.3389/fonc.2020.00486.
Yeo CD, Kang N, Choi SY, Kim BN, Park CK, et al. The role of hypoxia on the acquisition of epithelial-mesenchymal transition and cancer stemness: a possible link to epigenetic regulation. Korean J Intern Med. 2017;32(4):589–99. https://doi.org/10.3904/kjim.2016.302.
Kung-Chun Chiu D, Pui-Wah Tse A, Law CT, Ming-Jing Xu I, Lee D, et al. Hypoxia regulates the mitochondrial activity of hepatocellular carcinoma cells through HIF/HEY1/PINK1 pathway. Cell Death Dis. 2019;10(12):934. https://doi.org/10.1038/s41419-019-2155-3.
Liu Z, Tu K, Wang Y, Yao B, Li Q, et al. Hypoxia accelerates aggressiveness of hepatocellular carcinoma cells involving oxidative stress, epithelial-mesenchymal transition and non-canonical hedgehog signaling. Cell Physiol Biochem. 2017;44(5):1856–68. https://doi.org/10.1159/000485821.
Lv C, Wang S, Lin L, Wang C, Zeng K, et al. USP14 maintains HIF1-alpha stabilization via its deubiquitination activity in hepatocellular carcinoma. Cell Death Dis. 2021;12(9):803. https://doi.org/10.1038/s41419-021-04089-6.
Yan G, Wang X, Sun C, Zheng X, Wei H, et al. Chronic alcohol consumption promotes diethylnitrosamine-induced hepatocarcinogenesis via immune disturbances. Sci Rep. 2017;7(1):2567. https://doi.org/10.1038/s41598-017-02887-7.
Mitra A, Yan J, Xia X, Zhou S, Chen J, et al. IL6-mediated inflammatory loop reprograms normal to epithelial-mesenchymal transition(+) metastatic cancer stem cells in preneoplastic liver of transforming growth factor beta-deficient beta2-spectrin(+/-) mice. Hepatology. 2017;65(4):1222–36. https://doi.org/10.1002/hep.28951.
Bergmann J, Muller M, Baumann N, Reichert M, Heneweer C, et al. IL-6 trans-signaling is essential for the development of hepatocellular carcinoma in mice. Hepatology. 2017;65(1):89–103. https://doi.org/10.1002/hep.28874.
Chen Y, Meng L, Shang H, Dou Q, Lu Z, et al. Beta2 spectrin-mediated differentiation repressed the properties of liver cancer stem cells through beta-catenin. Cell Death Dis. 2018;9(4):424. https://doi.org/10.1038/s41419-018-0456-6.
Deng YR, Ma HD, Tsuneyama K, Yang W, Wang YH, et al. STAT3-mediated attenuation of CCl4-induced mouse liver fibrosis by the protein kinase inhibitor sorafenib. J Autoimmun. 2013;46:25–34. https://doi.org/10.1016/j.jaut.2013.07.008.
Li Y, Chen G, Han Z, Cheng H, Qiao L, et al. IL-6/STAT3 Signaling contributes to sorafenib resistance in hepatocellular carcinoma through targeting cancer stem cells. Onco Targets Ther. 2020;13:9721–30. https://doi.org/10.2147/OTT.S262089.
Wael H, Yoshida R, Kudoh S, Hasegawa K, Niimori-Kita K, et al. Notch1 signaling controls cell proliferation, apoptosis and differentiation in lung carcinoma. Lung Cancer. 2014;85(2):131–40. https://doi.org/10.1016/j.lungcan.2014.05.001.
Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–89. https://doi.org/10.1038/nrm2009.
Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol. 2016;17(11):722–35. https://doi.org/10.1038/nrm.2016.94.
Allenspach EJ, Maillard I, Aster JC, Pear WS. Notch signaling in cancer. Cancer Biol Ther. 2002;1(5):466–76. https://doi.org/10.4161/cbt.1.5.159.
Aster JC, Pear WS, Blacklow SC. The varied roles of notch in cancer. Annu Rev Pathol. 2017;12:245–75. https://doi.org/10.1146/annurev-pathol-052016-100127.
Nowell CS, Radtke F. Notch as a tumour suppressor. Nat Rev Cancer. 2017;17(3):145–59. https://doi.org/10.1038/nrc.2016.145.
Zhu C, Ho YJ, Salomao MA, Dapito DH, Bartolome A, et al. Notch activity characterizes a common hepatocellular carcinoma subtype with unique molecular and clinicopathologic features. J Hepatol. 2021;74(3):613–26. https://doi.org/10.1016/j.jhep.2020.09.032.
Shen H, McElhinny AS, Cao Y, Gao P, Liu J, et al. The Notch coactivator, MAML1, functions as a novel coactivator for MEF2C-mediated transcription and is required for normal myogenesis. Genes Dev. 2006;20(6):675–88. https://doi.org/10.1101/gad.1383706.
Wang J, Zhang SM, Wu JM, Lu ZC, Yang JR, et al. Mastermind-like transcriptional coactivator 1 overexpression predicts poor prognosis in human with hepatocellular carcinoma. Ann Clin Lab Sci. 2016;46(5):502–7.
Zema S, Pelullo M, Nardozza F, Felli MP, Screpanti I, et al. A dynamic role of mastermind-like 1: a journey through the main (path)ways between development and cancer. Front Cell Dev Biol. 2020;8: 613557. https://doi.org/10.3389/fcell.2020.613557.
Saha SK, Choi HY, Yang GM, Biswas PK, Kim K, et al. GPR50 promotes hepatocellular carcinoma progression via the notch signaling pathway through direct interaction with ADAM17. Mol Ther Oncolytics. 2020;17:332–49. https://doi.org/10.1016/j.omto.2020.04.002.
Garg M. Epithelial-mesenchymal transition - activating transcription factors - multifunctional regulators in cancer. World J Stem Cells. 2013;5(4):188–95. https://doi.org/10.4252/wjsc.v5.i4.188.
Wang Z, Li Y, Kong D, Sarkar FH. The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets. 2010;11(6):745–51. https://doi.org/10.2174/138945010791170860.
Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23(5):1155–65. https://doi.org/10.1038/sj.emboj.7600069.
Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18(1):99–115. https://doi.org/10.1101/gad.276304.
Pires BR, Mencalha AL, Ferreira GM, de Souza WF, Morgado-Diaz JA, et al. NF-kappaB is involved in the regulation of EMT genes in breast cancer cells. PLoS ONE. 2017;12(1): e0169622. https://doi.org/10.1371/journal.pone.0169622.
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, et al. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15(5):416–28. https://doi.org/10.1016/j.ccr.2009.03.016.
Abbott DW, Laszczak M, Lewis JD, Su H, Moore SC, et al. Structural characterization of macroH2A containing chromatin. Biochemistry. 2004;43(5):1352–9. https://doi.org/10.1021/bi035859i.
Chang H, Li J, Qu K, Wan Y, Liu S, et al. CRIF1 overexpression facilitates tumor growth and metastasis through inducing ROS/NFkappaB pathway in hepatocellular carcinoma. Cell Death Dis. 2020;11(5):332. https://doi.org/10.1038/s41419-020-2528-7.
Jing Y, Han Z, Liu Y, Sun K, Zhang S, et al. Mesenchymal stem cells in inflammation microenvironment accelerates hepatocellular carcinoma metastasis by inducing epithelial-mesenchymal transition. PLoS ONE. 2012;7(8): e43272. https://doi.org/10.1371/journal.pone.0043272.
Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843(11):2563–82. https://doi.org/10.1016/j.bbamcr.2014.05.014.
Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944–71. https://doi.org/10.1111/febs.14466.
Huang F, Geng XP. Chemokines and hepatocellular carcinoma. World J Gastroenterol. 2010;16(15):1832–6. https://doi.org/10.3748/wjg.v16.i15.1832.
Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4(12):2171–85. https://doi.org/10.18632/oncotarget.1426.
Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–72. https://doi.org/10.1038/nri.2017.49.
Liang CM, Chen L, Hu H, Ma HY, Gao LL, et al. Chemokines and their receptors play important roles in the development of hepatocellular carcinoma. World J Hepatol. 2015;7(10):1390–402. https://doi.org/10.4254/wjh.v7.i10.1390.
Liu Q, Li A, Tian Y, Wu JD, Liu Y, et al. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016;31:61–71. https://doi.org/10.1016/j.cytogfr.2016.08.002.
Batlle R, Andres E, Gonzalez L, Llonch E, Igea A, et al. Regulation of tumor angiogenesis and mesenchymal-endothelial transition by p38alpha through TGF-beta and JNK signaling. Nat Commun. 2019;10(1):3071. https://doi.org/10.1038/s41467-019-10946-y.
Gunderson AJ, Yamazaki T, McCarty K, Fox N, Phillips M, et al. TGFbeta suppresses CD8(+) T cell expression of CXCR3 and tumor trafficking. Nat Commun. 2020;11(1):1749. https://doi.org/10.1038/s41467-020-15404-8.
Yang L. TGFbeta and cancer metastasis: an inflammation link. Cancer Metastasis Rev. 2010;29(2):263–71. https://doi.org/10.1007/s10555-010-9226-3.
Barashi N, Weiss ID, Wald O, Wald H, Beider K, et al. Inflammation-induced hepatocellular carcinoma is dependent on CCR5 in mice. Hepatology. 2013;58(3):1021–30. https://doi.org/10.1002/hep.26403.
Singh SK, Mishra MK, Rivers BM, Gordetsky JB, Bae S, et al. Biological and clinical significance of the CCR5/CCL5 axis in hepatocellular carcinoma. Cancers (Basel). 2020;12(4). https://doi.org/10.3390/cancers12040883.
Kopitar-Jerala N. The role of interferons in inflammation and inflammasome activation. Front Immunol. 2017;8:873. https://doi.org/10.3389/fimmu.2017.00873.
Walter MR. The role of structure in the biology of interferon signaling. Front Immunol. 2020;11: 606489. https://doi.org/10.3389/fimmu.2020.606489.
Rauch I, Muller M, Decker T. The regulation of inflammation by interferons and their STATs. JAKSTAT. 2013;2(1): e23820. https://doi.org/10.4161/jkst.23820.
Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–86. https://doi.org/10.1038/nri1604.
Wang H, Brown J, Garcia CA, Tang Y, Benakanakere MR, et al. The role of glycogen synthase kinase 3 in regulating IFN-beta-mediated IL-10 production. J Immunol. 2011;186(2):675–84. https://doi.org/10.4049/jimmunol.1001473.
Romerio F, Riva A, Zella D. Interferon-alpha2b reduces phosphorylation and activity of MEK and ERK through a Ras/Raf-independent mechanism. Br J Cancer. 2000;83(4):532–8. https://doi.org/10.1054/bjoc.2000.1263.
Yeh YH, Hsiao HF, Yeh YC, Chen TW, Li TK. Inflammatory interferon activates HIF-1alpha-mediated epithelial-to-mesenchymal transition via PI3K/AKT/mTOR pathway. J Exp Clin Cancer Res. 2018;37(1):70. https://doi.org/10.1186/s13046-018-0730-6.
Herzer K, Hofmann TG, Teufel A, Schimanski CC, Moehler M, et al. IFN-alpha-induced apoptosis in hepatocellular carcinoma involves promyelocytic leukemia protein and TRAIL independently of p53. Cancer Res. 2009;69(3):855–62. https://doi.org/10.1158/0008-5472.CAN-08-2831.
Zhang Z, Zhu Y, Xu D, Li TE, Li JH, et al. IFN-alpha facilitates the effect of sorafenib via shifting the M2-like polarization of TAM in hepatocellular carcinoma. Am J Transl Res. 2021;13(1):301–13.
Wang H, Liu J, Hu X, Liu S, He B. Prognostic and therapeutic values of tumor necrosis factor-alpha in hepatocellular carcinoma. Med Sci Monit. 2016;22:3694–704. https://doi.org/10.12659/msm.899773.
Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101(2):311–20. https://doi.org/10.1172/JCI1368.
Popko K, Gorska E, Stelmaszczyk-Emmel A, Plywaczewski R, Stoklosa A, et al. Proinflammatory cytokines Il-6 and TNF-alpha and the development of inflammation in obese subjects. Eur J Med Res. 2010;15(Suppl 2):120–2. https://doi.org/10.1186/2047-783x-15-s2-120.
Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28(33):2940–7. https://doi.org/10.1038/onc.2009.180.
Wendt MK, Balanis N, Carlin CR, Schiemann WP. STAT3 and epithelial-mesenchymal transitions in carcinomas. JAKSTAT. 2014;3(1): e28975. https://doi.org/10.4161/jkst.28975.
Gyamfi J, Lee YH, Eom M, Choi J. Interleukin-6/STAT3 signalling regulates adipocyte induced epithelial-mesenchymal transition in breast cancer cells. Sci Rep. 2018;8(1):8859. https://doi.org/10.1038/s41598-018-27184-9.
Lin RJ, Afshar-Kharghan V, Schafer AI. Paraneoplastic thrombocytosis: the secrets of tumor self-promotion. Blood. 2014;124(2):184–7. https://doi.org/10.1182/blood-2014-03-562538.
Porta C, De Amici M, Quaglini S, Paglino C, Tagliani F, et al. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. 2008;19(2):353–8. https://doi.org/10.1093/annonc/mdm448.
Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA, Jr. Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am J Pathol. 1985;121(3):394–403.
Faletti L, Peintner L, Neumann S, Sandler S, Grabinger T, et al. TNFalpha sensitizes hepatocytes to FasL-induced apoptosis by NFkappaB-mediated Fas upregulation. Cell Death Dis. 2018;9(9):909. https://doi.org/10.1038/s41419-018-0935-9.
Wang Y, Xu J, Zhang X, Wang C, Huang Y, et al. TNF-alpha-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death Dis. 2017;8(3): e2715. https://doi.org/10.1038/cddis.2017.129.
Wang H, Wang HS, Zhou BH, Li CL, Zhang F, et al. Epithelial-mesenchymal transition (EMT) induced by TNF-alpha requires AKT/GSK-3beta-mediated stabilization of snail in colorectal cancer. PLoS ONE. 2013;8(2): e56664. https://doi.org/10.1371/journal.pone.0056664.
Jang MK, Kim HS, Chung YH. Clinical aspects of tumor necrosis factor-alpha signaling in hepatocellular carcinoma. Curr Pharm Des. 2014;20(17):2799–808. https://doi.org/10.2174/13816128113199990587.
Page MJ, Bester J, Pretorius E. The inflammatory effects of TNF-alpha and complement component 3 on coagulation. Sci Rep. 2018;8(1):1812. https://doi.org/10.1038/s41598-018-20220-8.
Neophytou, C.M., M. Panagi, T. Stylianopoulos, and P. Papageorgis, The role of tumor microenvironment in cancer metastasis: molecular mechanisms and therapeutic opportunities. Cancers (Basel), 2021. 13(9). https://doi.org/10.3390/cancers13092053.
Kim J, Bae JS. Tumor-Associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:6058147. https://doi.org/10.1155/2016/6058147.
Shirabe K, Mano Y, Muto J, Matono R, Motomura T, et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg Today. 2012;42(1):1–7. https://doi.org/10.1007/s00595-011-0058-8.
Li Z, Wu T, Zheng B, Chen L. Individualized precision treatment: targeting TAM in HCC. Cancer Lett. 2019;458:86–91. https://doi.org/10.1016/j.canlet.2019.05.019.
Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013: 187204. https://doi.org/10.1155/2013/187204.
Faurobert E, Bouin AP, Albiges-Rizo C. Microenvironment, tumor cell plasticity, and cancer. Curr Opin Oncol. 2015;27(1):64–70. https://doi.org/10.1097/CCO.0000000000000154.
Runa F, Hamalian S, Meade K, Shisgal P, Gray PC, et al. Tumor microenvironment heterogeneity: challenges and opportunities. Curr Mol Biol Rep. 2017;3(4):218–29. https://doi.org/10.1007/s40610-017-0073-7.
Cao MX, Jiang YP, Tang YL, Liang XH. The crosstalk between lncRNA and microRNA in cancer metastasis: orchestrating the epithelial-mesenchymal plasticity. Oncotarget. 2017;8(7):12472–83. https://doi.org/10.18632/oncotarget.13957.
Jin K, Li T, Sanchez-Duffhues G, Zhou F, Zhang L. Involvement of inflammation and its related microRNAs in hepatocellular carcinoma. Oncotarget. 2017;8(13):22145–65. https://doi.org/10.18632/oncotarget.13530.
Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28(40):3526–36. https://doi.org/10.1038/onc.2009.211.
Ha SY, Yu JI, Choi C, Kang SY, Joh JW, et al. Prognostic significance of miR-122 expression after curative resection in patients with hepatocellular carcinoma. Sci Rep. 2019;9(1):14738. https://doi.org/10.1038/s41598-019-50594-2.
Yu X, Zheng H, Chan MT, Wu WK. HULC: an oncogenic long non-coding RNA in human cancer. J Cell Mol Med. 2017;21(2):410–7. https://doi.org/10.1111/jcmm.12956.
Li SP, Xu HX, Yu Y, He JD, Wang Z, et al. LncRNA HULC enhances epithelial-mesenchymal transition to promote tumorigenesis and metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1 signaling pathway. Oncotarget. 2016;7(27):42431–46. https://doi.org/10.18632/oncotarget.9883.
Sphyris N, Mani SA. pIgR: frenemy of inflammation, EMT, and HCC progression. J Natl Cancer Inst. 2011;103(22):1644–5. https://doi.org/10.1093/jnci/djr421.
Ai J, Tang Q, Wu Y, Xu Y, Feng T, et al. The role of polymeric immunoglobulin receptor in inflammation-induced tumor metastasis of human hepatocellular carcinoma. J Natl Cancer Inst. 2011;103(22):1696–712. https://doi.org/10.1093/jnci/djr360.
Critelli R, Milosa F, Faillaci F, Condello R, Turola E, et al. Microenvironment inflammatory infiltrate drives growth speed and outcome of hepatocellular carcinoma: a prospective clinical study. Cell Death Dis. 2017;8(8): e3017. https://doi.org/10.1038/cddis.2017.395.
Zhang Y, Zhang J, Chen X, Yang Z. Polymeric immunoglobulin receptor (PIGR) exerts oncogenic functions via activating ribosome pathway in hepatocellular carcinoma. Int J Med Sci. 2021;18(2):364–71. https://doi.org/10.7150/ijms.49790.
Ben Mousa A. Sorafenib in the treatment of advanced hepatocellular carcinoma. Saudi J Gastroenterol. 2008;14(1):40–2. https://doi.org/10.4103/1319-3767.37808.
Lv X, Fang C, Yin R, Qiao B, Shang R, et al. Agrin para-secreted by PDGF-activated human hepatic stellate cells promotes hepatocarcinogenesis in vitro and in vivo. Oncotarget. 2017;8(62):105340–55. https://doi.org/10.18632/oncotarget.22186.
Zhang QJ, Wan L, Xu HF. High expression of agrin is associated with tumor progression and poor prognosis in hepatocellular carcinoma. Math Biosci Eng. 2019;16(6):7375–83. https://doi.org/10.3934/mbe.2019368.
Lozano-Ruiz B, Gonzalez-Navajas JM. The emerging relevance of AIM2 in liver disease. Int J Mol Sci. 2020;21(18). https://doi.org/10.3390/ijms21186535.
Chen SL, Liu LL, Lu SX, Luo RZ, Wang CH, et al. HBx-mediated decrease of AIM2 contributes to hepatocellular carcinoma metastasis. Mol Oncol. 2017;11(9):1225–40. https://doi.org/10.1002/1878-0261.12090.
Funding
This work was supported in part by NIH grant number CA 82723 (to BIC) and TÜBİTAK grant numbers 114Z245, 117Z223, 120N556, and 219Z034 (to H.A).
Author information
Authors and Affiliations
Contributions
Conceptualization, H. A. and B. I. C.; writing—original draft preparation, B. S.; writing—review and editing, B. I. C and H. A.; funding acquisition, B. I. C. and H. A. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sengez, B., Carr, B.I. & Alotaibi, H. EMT and Inflammation: Crossroads in HCC. J Gastrointest Canc 54, 204–212 (2023). https://doi.org/10.1007/s12029-021-00801-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-021-00801-z