Abstract
Background
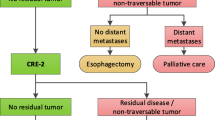
Response assessment after chemo-radiotherapy (CTRT) in locally advanced esophageal cancer is usually performed using a PET-CT scan, an upper GI endoscopy (UGIE) and histological correlation with biopsy or cytology. We aim to study the incremental value of brush cytology in addition to PET-CT for response assessment.
Materials and methods
In this retrospective analysis, 40 patients with Stage II- IV carcinoma esophagus treated with radical intent between June 2015 and August 2019 were included. Patients were treated with either upfront concurrent CTRT or neo-adjuvant chemotherapy followed by CTRT. All patients underwent PET-CT and UGIE for initial staging and response assessment on follow-up. Patients with esophageal stricture (disease related or treatment induced) had brush cytology done during UGIE. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of brush cytology were calculated considering serial clinical follow-up as gold standard.
Results
Twenty-three male (57.5%) and 17 (42.5%) female patients with median age of 57 years (range: 27 – 79 years) were analyzed. Concurrent CTRT was delivered in 52.5%; 75% patients were treated with intensity-modulated radiotherapy (IMRT); median RT dose was 63 Gy (range- 41.4 to 64 Gy). At a median follow-up of 16 months (range 6- 54 months), 20 patients (55.5%) were clinically controlled, 9 (25%) had local recurrence, 5 (13.8%) had loco-regional recurrence and 2 had distant metastasis. Considering clinical follow-up as the gold standard, sensitivity, PPV and NPV of PET-CT combined with brush cytology improved compared to PET-CT alone and was found to be 75%, 90%, 85.7% and 81.8% respectively.
Conclusion
We found that brush cytology on endoscopy is a simple tool with high specificity which adds value to the findings of response assessment PET-CT scan and thereby can increase the confidence of the treating oncologist in making clinical decisions.


Similar content being viewed by others
Data Availability
Yes, on request.
References
Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JAJ, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01). Radiation Therapy Oncology Group JAMA. 1999;281(17):1623–7.
Wu AJ, Goodman KA. Clinical tools to predict outcomes in patients with esophageal cancer treated with definitive chemoradiation: Are we there yet? J GastrointestOncol. 2015;6(1):53–9.
Swisher SG, Maish M, Erasmus JJ, Correa AM, Ajani JA, Bresalier R, et al. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann ThoracSurg. 2004;78(4):1152–60.
Bruzzi JF, Munden RF, Truong MT, Marom EM, Sabloff BS, Gladish GW, et al. PET/CT of Esophageal Cancer: Its Role in Clinical Management. RadioGraphics. 2007;27(6):1635–52.
Jayachandran P, Pai RK, Quon A, Graves E, Krakow TE, La T, et al. Postchemoradiotherapy positron emission tomography predicts pathologic response and survival in patients with esophageal cancer. Int J RadiatOncolBiolPhys. 2012;84(2):471–7.
Flamen P, Van Cutsem E, Lerut A, Cambier JP, Haustermans K, Bormans G, et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol. 2002;13(3):361–8.
Roedl JB, Harisinghani MG, Colen RR, Fischman AJ, Blake MA, Mathisen DJ, et al. Assessment of treatment response and recurrence in esophageal carcinoma based on tumor length and standardized uptake value on positron emission tomography-computed tomography. Ann ThoracSurg. 2008;86(4):1131–8.
D’Journo X-B, Michelet P, Dahan L, Doddoli C, Seitz J-F, Giudicelli R, et al. Indications and outcome of salvage surgery for oesophageal cancer. Eur J CardiothoracSurg. 2008;33(6):1117–23.
Kaur S, Sharma R, Kaushal V, Gulati A, Sharma B. Diagnostic accuracy of endoscopic brush cytology in malignancies of upper gastrointestinal tract: A prospective study of 251 patients in North India. J Cancer Res Ther. 2016;12(2):681–4.
Bakheet SM, Saleem M, Powe J, Al-Amro A, Larsson SG, Mahassin Z. F-18 fluorodeoxyglucose chest uptake in lung inflammation and infection. ClinNucl Med. 2000;25(4):273–8.
Bhargava P, Reich P, Alavi A, Zhuang H. Radiation-Induced Esophagitis on FDG PET Imaging: Clinical Nuclear Medicine. 2003;28(10):849–50.
Swisher SG, Marks J, Rice D. Salvage esophagectomy for persistent or recurrent disease after definitive chemoradiation. Ann CardiothoracSurg. 2017;6(2):144–51.
Kiyozumi Y, Yoshida N, Ishimoto T, Yagi T, Koga Y, Uchihara T, et al. Prognostic Factors of Salvage Esophagectomy for Residual or Recurrent Esophageal Squamous Cell Carcinoma After Definitive Chemoradiotherapy. World J Surg. 2018;42(9):2887–93.
Peng HQ, Halsey K, Sun CCJ, Manucha V, Nugent S, Rodgers WH, et al. Clinical utility of postchemoradiation endoscopic brush cytology and biopsy in predicting residual esophageal adenocarcinoma. Cancer Cytopathol. 2009;117(6):463–72.
O’Donoghue JM, Horgan PG, O’Donohoe MK, Byrne J, O’Hanlon DM, McGuire M, et al. Adjunctive endoscopic brush cytology in the detection of upper gastrointestinal malignancy. ActaCytol. 1995;39(1):28–34.
Cook IJ, de Carle DJ, Haneman B, Hunt DR, Talley NA, Miller D. The role of brushing cytology in the diagnosis of gastric malignancy. ActaCytol. 1988;32(4):461–4.
Vidyavathi K, Harendrakumar M, Lakshmana KY. Correlation of endoscopic brush cytology with biopsy in diagnosis of upper gastrointestinal neoplasms. Indian J PatholMicrobiol. 2008;51(4):489–92.
Geramizadeh B, Shafiee A, Saberfirruzi M, Kumar PK, Shaheem A. Brush cytology of gastric malignancies. ActaCytol. 2002;46(4):693–6.
Hamadi SS. Endoscopic biopsy and brushing cytology compared to open tissue biopsy , a study of 50 patients with upper gastrointestinal tract malignancy. 2019;(September).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
Approved by institutional IRB.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karmakar, S., Mummudi, N., Ghosh-Laskar, S. et al. Incremental value of endoscopic brush cytology in response assessment after chemo-irradiation for Esophageal cancer. J Gastrointest Canc 53, 122–129 (2022). https://doi.org/10.1007/s12029-020-00555-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-020-00555-0