Abstract
Background
Abdominal and back pain is present in up to 80% of patients with pancreatic cancer and represents a significant cause of morbidity. Celiac plexus neurolysis (CPN) demonstrated good results in relief of pain of upper abdominal malignancy. Dexmedetomidine is alpha-2 adrenoceptor highly selective agonist approved for procedural sedation use.
Patients and Methods
Fifty patients divided in two groups with locally advanced pancreatic cancer-associated abdominal pain underwent endoscopic ultrasound (EUS)-guided CPN using bupivacaine 0.5% alone with alcohol for the first group and bupivacaine 0.5% plus dexmedetomidine in the second. Patients scored their pain according to the Numeric Rating Scale (NRS-11) before, 2, 4, 6, 8, 12, 16, and 24 week after the procedure.
Results
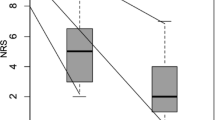
The study has included 50 patient in two groups. There was no significant difference between the two groups as regards medical, laboratory, or tumor characters. The median pain score decreases from 8.32 ± 0.75 before the procedure to 3.75 ± 3.72 24 week after the procedure in group 1 and from 8.08 ± 0.86 before to 1.67 ± 2.3 24 week after the procedure in group 2. However, there was no significant difference between the two groups in the median pain score during the first 4 weeks. There was no statistically significant difference between the two groups as regards the median survival time.
Conclusion
The addition of dexmedetomidine to bupivacaine 0.5% in EUS-CPN demonstrated beneficial effects as regards the degree and duration of pain relieve with negligible effect on the patient survival.


Similar content being viewed by others
Data Availability
Available at the data archive at the Specialized Medical Hospital, Faculty of Medicine, Mansoura University, Egypt.
References
Sirri E, Castro FA, Kieschke J, Jansen L, Emrich K, Gondos A, et al. Recent trends in survival of patients with pancreatic cancer in Germany and the United States. Pancreas. 2016;45:908–14.
Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011. https://doi.org/10.1002/14651858.CD007519.pub2.
Jooste V, Grosclaude P, Remontet L, Launoy G, Baldi I, Molinié F, et al. Unbiased estimates of long-term net survival of solid cancers in France. Int J Cancer. 2013;132:2370–7.
Vranken JH, Zuurmond WW, Van Kemenade FJ, Dzoljic M. Neurohistopathologic findings after a neurolytic celiac plexus block with alcohol in patients with pancreatic cancer pain. Acta Anaesthesiol Scand. 2002;46:827–30.
Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291:1092–9.
Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis - a practical guide. Aliment Pharmacol Ther. 2013;37:1132–56.
Eloubeidi MA. Initial evaluation of the forward-viewing echoendoscope prototype for performing fine-needle aspiration, Tru-cut biopsy and celiac plexus neurolysis. J Gastroenterol Hepatol. 2011;26:63–7.
Sakamoto H, Kitano M, Kamata K, Komaki T, Imai H, Chikugo T, et al. EUS-guided broad plexus neurolysis over the superior mesenteric artery using a25-gauge needle. Am J Gastroenterol. 2010;105:2599–606.
Ha TI, Kim GH, Kang DH, Song GA, Kim S, Lee JW. Detection of celiac ganglia with radial scanning endoscopic ultrasonography. Korean J Intern Med. 2008;23:5–8.
Callado LF, Stamford JA. Alpha-2A but not alpha-2B/C adrenoceptors modulate noradrenaline release in rat locus coeruleus: voltammetric data. Eur J Pharmacol. 1999;366:35–9.
Jones CR. Perioperative uses of dexmedetomidine. Int Anesthesiol Clin. 2013;51:81–96.
Piao G, Wu J. Systematic assessment of dexmedetomidine as an anesthetic agent: a meta-analysis of randomized controlled trials. Arch Med Sci. 2014;10:19–24.
Tobias JD. Dexmedetomidine: applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8:115–31.
Tug A, Hanci A, Turk HS, Avbey F, Isil CT, Savin P, et al. Comparison of two different intranasal doses of dexmedetomidine in children for magnetic resonance imaging sedation. Paediatr Drugs. 2015;17:479–85.
Hilliard N, Brown S, Mitchinson S. A case report of dexmedetomidine used to treat intractable pain and delirium in a tertiary palliative care unit. Palliat Med. 2015;29:278–81.
Cimen ZS, Hanci A, Sivrikaya GU, Kilinc LT, Erol MK. Comparison of buccal and nasal dexmedetomidine premedication for pediatric patients. Paediatr Anaesth. 2013;23:134–8.
Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2013;110:915–25.
Gertle R, Brown C, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. BUMC Proc. 2001;14:13–21.
Nagels W, Pease N, Bekkering G, Cools F, Dobbels P. Celiac plexus neurolysis for abdominal cancer pain: a systematic review. Pain Med. 2013;14:1140–63.
NCCN Guidelines for pancreatic adenocarcinoma (2017) Version 3.Availableonline:http://jaxelection.altervista.org/pancreatic/NCCN3.Pancreatic.pdf (accessed on 21 December 2017).
Levy MJ, Chari ST, Wiersema MJ. Endoscopic ultrasound guided celiac neurolysis. Gastrointest Endosc Clin N Am. 2012;22:231–47.
Ramirez-Luna MA, Chavez-Tapia NC, Franco-Guzman AM, Garcia-Saenz-de Sicilia M, Tellez-Avila FI. Endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. Rev Gastroenterol Mex. 2008;73:63–7.
Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, et al. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–34.
Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330–7.
Birnbaumer L, Abramowitz J, Brown AM. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990;1031:163–224.
Jain PN, Shrikhande SV, Myatra SN, Sareen R. Neurolytic celiac plexus block: a better alternative to opioid treatment in upper abdominal malignancies: an Indian experience. J Pain Palliat Care Pharmacother. 2005;19:15–20.
Kawamata M, Ishitani K, Ishikawa K, Sasaki H, Ota K, Omote K, et al. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain. 1996;64:597–602.
LeBlanc JK, Al-Haddad M, McHenry L, Sherman S, Juan M, McGreevy K, et al. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. 2011;74:1300–7.
Acknowledgments
The authors would like to thank all patients who participated in the study.
Author information
Authors and Affiliations
Contributions
Ahmed Abdel Ghafar: choosing idea, patient examination, writing, reviewing
Ahmed sultan: patient examination, writing, reviewing
Mohamed Hammouda: software, reviewing
Ahmed Shawki: reviewing
Mohamed Abd El Ghaffar: patient referral
All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflicts of interest.
Ethics Approval and Consent to Participate
Written consents from patients participated in the study or from their families were obtained and approved by the Mansoura Medical Ethics Committee (MMEC) of Faculty of Medicine.
Consent for Publication
NA
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saleh, A.A.G., Sultan, A., Hammouda, M.A. et al. Value of Adding Dexmedetomidine in Endoscopic Ultrasound-Guided Celiac Plexus Neurolysis for Treatment of Pancreatic Cancer-Associated Pain. J Gastrointest Canc 52, 682–689 (2021). https://doi.org/10.1007/s12029-020-00449-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-020-00449-1