Abstract
Purpose
Congenital adrenal hyperplasia (CAH) is rare autosomal recessive disease. CAH due to 21-hydroxylase deficiency accounts for 95% of cases. We aimed to share the first case of coexistence of simple virilizing-type congenital adrenal hyperplasia [I172N mutation in the CYP21A], triple translocation [t(9;11;12)], and ovarian granulose cell tumor.
Methods
A 59-year-old female patient was presented to our clinic, complaining with abdominal pain and distension. Physical examination revealed palpable abdominal mass, virilism, ambiguous genitalia, clitoramegaly, and hyperpigmentation. Contrast-enhanced abdominal computed tomography showed a giant mass originating from the right tubo-ovarian structure.
Results
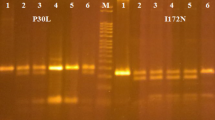
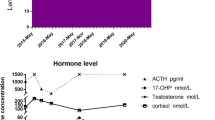
The patient was operated in the light of the clinico-radiological features mentioned above. A giant mass weighing 3500 g was detected on the right tubo-ovarian structure during laparotomy, and mass was excised with right tubo-ovarian structure. Immunohistochemical examination revealed ovarian granulosa cell tumor. The high serum concentration of 17-OH progesterone was measured at baseline and after 250-μg bolus of synthetic ACTH. In genetic analysis, we screened for six-point mutations, large deletions, and non-common mutations using restriction fragment length polymorphism (RFLP) methods, PCR, and sequencing of CYP21 gene respectively. The patient was detected to be homozygous for the I172N mutation. In addition, 50% of the metaphases examined had triple translocation [t(9;11;12)].
Conclusion
The coexistence of congenital adrenal hyperplasia, triple chromosomal translocations, and ovarian granulosa cell tumor has not been described previously. This coexistence may be a sign of a new syndrome.






Similar content being viewed by others

References
Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:4043–88. https://doi.org/10.1210/jc.2018-01865.
Nordenstrom A, Falhammar H. Diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2018. https://doi.org/10.1530/EJE-18-0712.
Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, et al. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95:5110–21. https://doi.org/10.1210/jc.2010-0917.
Faraj G, Di Gregorio S, Misiunas A, Faure EN, Villabrile P, Stringa I, et al. Virilizing ovarian tumor of cell tumor type not otherwise specified: a case report. Gynecol Endocrinol. 1998;12:347–52.
Khosla D, Dimri K, Pandey AK, Mahajan R, Trehan R. Ovarian granulosa cell tumor: clinical features, treatment, outcome, and prognostic factors. N Am J Med Sci. 2014;6:133–8. https://doi.org/10.4103/1947-2714.128475.
Chilkoti GT, Singh A, Mohta M, Saxena AK. Perioperative “stress dose” of corticosteroid: pharmacological and clinical perspective. J Anaesthesiol Clin Pharmacol. 2019;35:147–52. https://doi.org/10.4103/joacp.JOACP_242_17.
Miller SA, Dynes DD, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. https://doi.org/10.1093/nar/16.3.1215.
Sadeghi F, Yurur-Kutlay N, Berberoglu M, Cetinkaya E, Aycan Z, Kara C, et al. Identification of frequency and distribution of the nine most frequent mutations among patients with 21-hydroxylase deficiency in Turkey. J Pediatr Endocrinol Metab. 2008;21:781–7. https://doi.org/10.1515/jpem.2008.21.8.7810.
Wilson RC, Wei JQ, Cheng KC, Mercado AB, New MI. Rapid deoxyribonucleic acid analysis by allele-specific polymerase chain reaction for detection of mutations in the 21-hydroxylase gene. J Clin Endocrinol Metab. 1995;80:1635–40. https://doi.org/10.1210/jcem.80.5.7745011.
Paulino L, De Araujo M, Guerra G Jr, Marini SHLV, De Mello MP. Mutation distribution and CYP21/C4 locus variability in Brazilian families with the classical form of the 21-hydroxylase deficiency. Acta Paediatr. 1999;88:275–83. https://doi.org/10.1080/08035259950170024.
Dingli D, Traulsen A, Lenaerts T, Pacheco JM. Evolutionary dynamics of chronic myeloid leukemia. Genes Cancer. 2010;1:309–15. https://doi.org/10.1177/1947601910371122.
Gidlof S, Falhammar H, Thilen A, von Dobeln U, Ritzen M, Wedell A, et al. One hundred years of congenital adrenal hyperplasia in Sweden: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol. 2013;1:35–42. https://doi.org/10.1016/S2213-8587(13)70007-X9.
Ambroziak U, Bednarczuk T, Ginalska-Malinowska M, Małunowicz EM, Grzechocinska B, Kaminski P, et al. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency - management in adults. Endokrynol Pol. 2010;61:142–55.
Imamine R, Arima H, Kusakabe M, Umeda H, Sato I, Homma K, et al. Genetic analysis of two Japanese patients with non-classical 21-hydroxylase deficiency. Intern Med. 2009;48:705–9. https://doi.org/10.2169/internalmedicine.48.1894.
White PC, Bachega TA. Congenital adrenal hyperplasia due to 21 hydroxylase deficiency: from birth to adulthood. Semin Reprod Med. 2012;30:400–9. https://doi.org/10.1055/s-0032-1324724.
Chen HD, Huang LE, Zhong ZH, Su Z, Jiang H, Zeng J, et al. Ovarian adrenal rest tumors undetected by imaging studies and identified at surgery in three females with congenital adrenal hyperplasia unresponsive to increased hormone therapy dosage. Endocr Pathol. 2017;28:146–51. https://doi.org/10.1007/s12022-016-9461-4.
Tiosano D, Vlodavsky E, Filmar S, Weiner Z, Goldsher D, Bar-Shalom R. Ovarian adrenal rest tumor in a congenital adrenal hyperplasia patient with adrenocorticotropin hypersecretion following adrenalectomy. Horm Res Paediatr. 2010;74:223–8. https://doi.org/10.1159/000295722.
Zaarour MG, Atallah DM, Trak-Smayra VE, Halaby GH. Bilateral ovary adrenal rest tumor in a congenital adrenal hyperplasia following adrenalectomy. Endocr Pract. 2014;20:e69–74. https://doi.org/10.4158/EP13092.CR.
Lim D, Oliva E. Ovarian sex cord-stromal tumours: an update in recent molecular advances. Pathology. 2018;50:178–89. https://doi.org/10.1016/j.pathol.2017.10.008.
Patel SS, Carrick KS, Carr BR. Virilization persists in a woman with an androgen-secreting granulosa cell tumor. Fertil Steril. 2009;91:933.e13–5. https://doi.org/10.1016/j.fertnstert.2008.10.038.
Gainder S, Kaur J, Siwatch S, Gupta N. Adult granulosa cell tumor: a sinister differential for clomiphene-resistant infertility. J Hum Reprod Sci. 2018;11:190–2. https://doi.org/10.4103/jhrs.JHRS_142_17.
Fuller PJ, Leung D, Chu S. Genetics and genomics of ovarian sex cord-stromal tumors. Clin Genet. 2017;91:285–91. https://doi.org/10.1111/cge.12917.
Author information
Authors and Affiliations
Contributions
Akbulut S, Arikan S, and Tuncali T contributed to this paper; Akbulut S and Arikan S designed the overall concept and outline of the manuscript; Akbulut S contributed to the writing and editing of the manuscript, illustrations, and review of literature; Sogutcu N provided histopathological examination.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not needed
Informed Consent Statement
Written consent was obtained from the patient.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akbulut, S., Ceylan, S.D., Tuncali, T. et al. Coexistence of Ovarian Granulose Cell Tumor, Congenital Adrenal Hyperplasia, and Triple Translocation: Is a Consequence or Coincidence?. J Gastrointest Canc 52, 508–514 (2021). https://doi.org/10.1007/s12029-020-00408-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-020-00408-w