Abstract
Background
Lynch syndrome (LS) increases the risk of many types of cancer, mainly colorectal cancer (CRC). The purpose of this study was to assess the prevalence of mismatch repair (MMR) deficiency in patients under the age of 50 with advanced adenomatous polyps, aiming at an early diagnosis of LS.
Methods
This retrospective, cross-sectional study included eligible patients with advanced adenomas diagnosed ≤ 50 years of age registered between April 2014 and February 2017 at three pathology centers in Mashhad. Pathological records were reviewed, and colon tissue specimens were analyzed by immunohistochemistry (IHC) staining to identify proteins which serve as markers for LS as they are related to loss of MMR gene (MLH1, MSH2, MSH6, and PMS2) expression.
Results
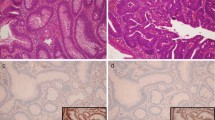
Of 862 consecutive patients, a total of 50 adenomas (54% males, 46% females of mean age 41.24 ± 6.5) met the eligibility criteria. Of the adenomas examined, 20 (40%) had a tubulovillous component, 34 (68%) had high-grade dysplasia, and 30 (60%) had were larger than 10 mm protrusions. None of the patients had loss of MMR protein expression.
Conclusion
No individual with MMR genetic disorder was identified by IHC screening of early-onset advanced colorectal adenomas. This strategy is therefore not an effective strategy for detecting MMR mutation carriers.

Similar content being viewed by others
Availability of Data and Material
Data will be available upon request.
References
Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US multi-society task force on colorectal cancer. Gastroenterology. 2014;147(2):502–26. https://doi.org/10.1053/j.gastro.2014.04.001.
Giardiello FM, Brensinger JD, Petersen GM. AGA technical review on hereditary colorectal cancer and genetic testing. Gastroenterology. 2001;121(1):198–213. https://doi.org/10.1053/gast.2001.25581.
Jass JR, Stewart SM, Stewart J, Lane MR. Hereditary non-polyposis colorectal cancer--morphologies, genes and mutations. Mutat Res. 1994;310(1):125–33. https://doi.org/10.1016/0027-5107(94)90016-7.
Lynch HT, Smyrk TC, Watson P, Lanspa SJ, Lynch JF, Lynch PM, et al. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology. 1993;104(5):1535–49. https://doi.org/10.1016/0016-5085(93)90368-m.
Mendelsohn RB, Herzog K, Shia J, Rahaman N, Stadler ZK, Shike M. Molecular screening for Lynch syndrome in young patients with colorectal adenomas. Clin Colorectal Cancer. 2017;16(3):173–7. https://doi.org/10.1016/j.clcc.2017.01.002.
Molaei M, Yadollahzadeh M, Almasi S, Shivarani S, Fatemi SR, Zali MR. Sporadic colorectal polyps and mismatch repair proteins. Indian J Pathol Microbiol. 2011;54(4):725–9. https://doi.org/10.4103/0377-4929.91505.
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–8. https://doi.org/10.1093/jnci/djh034.
Conteduca V, Sansonno D, Russi S, Dammacco F. Precancerous colorectal lesions (review). Int J Oncol. 2013;43(4):973–84. https://doi.org/10.3892/ijo.2013.2041.
Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017;3(4):464–71. https://doi.org/10.1001/jamaoncol.2016.5194.
Pino MS, Mino-Kenudson M, Wildemore BM, Ganguly A, Batten J, Sperduti I, et al. Deficient DNA mismatch repair is common in Lynch syndrome-associated colorectal adenomas. J Mol Diagn. 2009;11(3):238–47. https://doi.org/10.2353/jmoldx.2009.080142.
Tanaka M, Nakajima T, Sugano K, Yoshida T, Taniguchi H, Kanemitsu Y, et al. Mismatch repair deficiency in Lynch syndrome-associated colorectal adenomas is more prevalent in older patients. Histopathology. 2016;69(2):322–8. https://doi.org/10.1111/his.12941.
Walsh MD, Buchanan DD, Pearson SA, Clendenning M, Jenkins MA, Win AK, et al. Immunohistochemical testing of conventional adenomas for loss of expression of mismatch repair proteins in Lynch syndrome mutation carriers: a case series from the Australasian site of the colon cancer family registry. Mod Pathol. 2012;25(5):722–30. https://doi.org/10.1038/modpathol.2011.209.
Kushnir VM, Nalbantoglu I, Watson R, Goodwin J, Safar E, Chokshi RV, et al. Advanced colorectal adenomas in patients under 45 years of age are mostly sporadic. Dig Dis Sci. 2014;59(11):2757–64. https://doi.org/10.1007/s10620-014-3245-9.
De Jong AE, Morreau H, Van Puijenbroek M, Eilers PH, Wijnen J, Nagengast FM, et al. The role of mismatch repair gene defects in the development of adenomas in patients with HNPCC. Gastroenterology. 2004;126(1):42–8. https://doi.org/10.1053/j.gastro.2003.10.043.
Vasen HF, Taal BG, Nagengast FM, Griffioen G, Menko FH, Kleibeuker JH, et al. Hereditary nonpolyposis colorectal cancer: results of long-term surveillance in 50 families. Eur J Cancer. 1995;31a(7-8):1145–8.
Goshayeshi L, Ghaffarzadegan K, Khooei A, Esmaeilzadeh A, Rahmani Khorram M, Mosannen Mozaffari H, et al. Prevalence and clinicopathological characteristics of mismatch repair-deficient colorectal carcinoma in early onset cases as compared with late-onset cases: a retrospective cross-sectional study in northeastern Iran. BMJ Open. 2018;8(8):e023102. https://doi.org/10.1136/bmjopen-2018-023102.
Goshayeshi L, Khooiee A, Ghaffarzadegan K, Rahmani Khorram M, Bishehsari F, Hoseini B, Akhavan Rezayat K, Esmaeilzadeh A, Mosannen Mozaffari H, Ghanayee O, Lari S, Bahari A, Allahyari A, Bari A, Ganji A, Goshayeshi L, Rajabzadeh F, Esmaeili J (2017) Screening for Lynch syndrome in cases with colorectal carcinoma from Mashhad. Arch Iran Med 20 (6):332-337. 0172006/AIM.003.
Lee EJ, Park CK, Kim JW, Chang DK, Kim KM. Deletion mutation of BRAF in a serrated adenoma from a patient with familial adenomatous polyposis. APMIS. 2007;115(8):982–6. https://doi.org/10.1111/j.1600-0463.2007.apm_670.x.
Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10(2):486–9. https://doi.org/10.5812/ijem.3505.
Goverde A, Wagner A, Bruno MJ, Hofstra RMW, Doukas M, van der Weiden MM, et al. Routine molecular analysis for Lynch syndrome among adenomas or colorectal cancer within a National Screening Program. Gastroenterology. 2018;155(5):1410–5. https://doi.org/10.1053/j.gastro.2018.07.029.
Velayos FS, Allen BA, Conrad PG, Gum J Jr, Kakar S, Chung DC, et al. Low rate of microsatellite instability in young patients with adenomas: reassessing the Bethesda guidelines. Am J Gastroenterol. 2005;100(5):1143–9. https://doi.org/10.1111/j.1572-0241.2005.40862.x.
Jarvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomaki P, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118(5):829–34. https://doi.org/10.1016/s0016-5085(00)70168-5.
Jarvinen HJ, Renkonen-Sinisalo L, Aktan-Collan K, Peltomaki P, Aaltonen LA, Mecklin JP. Ten years after mutation testing for Lynch syndrome: cancer incidence and outcome in mutation-positive and mutation-negative family members. J Clin Oncol. 2009;27(28):4793–7. https://doi.org/10.1200/jco.2009.23.7784.
Stupart DA, Goldberg PA, Algar U, Ramesar R. Surveillance colonoscopy improves survival in a cohort of subjects with a single mismatch repair gene mutation. Colorectal Dis. 2009;11(2):126–30. https://doi.org/10.1111/j.1463-1318.2008.01702.x.
Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, Khan PM, et al. A National Cancer Institute Workshop on hereditary nonpolyposis colorectal cancer syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89(23):1758–62. https://doi.org/10.1093/jnci/89.23.1758.
Loukola A, Salovaara R, Kristo P, Moisio AL, Kaariainen H, Ahtola H, et al. Microsatellite instability in adenomas as a marker for hereditary nonpolyposis colorectal cancer. Am J Pathol. 1999;155(6):1849–53. https://doi.org/10.1016/s0002-9440(10)65503-4.
Zhu F, Pan D, Zhang H, Ye Q, Xu P, Pan J. Single-center study of Lynch syndrome screening in colorectal polyps. Hered Cancer Clin Pract. 2019;17:9. https://doi.org/10.1186/s13053-019-0108-6.
Goel A, Nagasaka T, Spiegel J, Meyer R, Lichliter WE, Boland CR. Low frequency of Lynch syndrome among young patients with non-familial colorectal cancer. Clin Gastroenterol Hepatol. 2010;8(11):966–71. https://doi.org/10.1016/j.cgh.2010.06.030.
Halvarsson B, Lindblom A, Johansson L, Lagerstedt K, Nilbert M. Loss of mismatch repair protein immunostaining in colorectal adenomas from patients with hereditary nonpolyposis colorectal cancer. Mod Pathol. 2005;18(8):1095–101. https://doi.org/10.1038/modpathol.3800392.
Shia J, Klimstra DS, Nafa K, Offit K, Guillem JG, Markowitz AJ, et al. Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J Surg Pathol. 2005;29(1):96–104.
Conway AA, Gerry JM, Sacco F, Wren SM. High prevalence of adenomatous polyps in Alaska native people aged 40-49 years. J Surg Res. 2019;243:524–30. https://doi.org/10.1016/j.jss.2019.07.004.
Acknowledgements
The authors would like to thank all study participants.
Funding
This study was funded by Mashhad University of Medical Sciences (Grant number 951047).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Benyamin Hoseini, Ladan Goshayeshi, and Mahla Rahmani Khorram.
The first draft of the manuscript was written by Mahla Rahmani Khorram and Fatemeh Maghool and then edited by Benyamin Hoseini. All authors commented on previous versions of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval and Consent to Participate
Ethical approval was obtained from the ethics committee of Mashhad University of Medical Sciences (IR.MUMS.fm.REC.1396.240), and the study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all participants before interviewing and/or testing.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khorram, M.R., Goshayeshi, L., Maghool, F. et al. Prevalence of Mismatch Repair-Deficient Colorectal Adenoma/Polyp in Early-Onset, Advanced Cases: a Cross-Sectional Study Based on Iranian Hereditary Colorectal Cancer Registry. J Gastrointest Canc 52, 263–268 (2021). https://doi.org/10.1007/s12029-020-00395-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-020-00395-y