Abstract
Purpose
Pancreatic adenocarcinoma remains a malignancy with poor prognosis. Black patients experience poorer overall survival compared with other races. Recent studies have elucidated certain prognostic factors at the time of diagnosis of pancreatic cancer which have largely not been studied for differences between racial groups. We present a study examining differences in blood levels between Black and non-Black patients and their effects on overall survival.
Methods
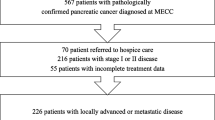
This is a retrospective cohort study. One hundred sixty-three patients were confirmed to carry a tissue diagnosis of pancreatic adenocarcinoma and included in analysis; 27 of the patients were self-identified as “Black”; 136 were analyzed together as “Non-Black” with the majority identifying as “White”. Various blood markers were drawn at the time of diagnosis. Kaplan-Meier and multivariable Cox regression models were used to examine differences in these factors between Black and non-Black patients, as well as their effect on overall survival.
Results
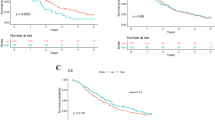
Black patients were younger at diagnosis (p = 0.001) and were more likely to experience significant weight loss leading up to diagnosis (p = 0.009); Black patients also had a lower neutrophil-to-lymphocyte ratio (NLR) (p = 0.001) and higher lymphocyte-to-monocyte ratio (LMR) (p = 0.001) at diagnosis. In multivariable analysis, an NLR > 3.5 had a significantly negative impact on overall survival (p = 0.002), as did the presence of metastatic disease (p < 0.001).
Conclusion
Black patients demonstrated a “favorable” white blood cell profile (higher LMR, lower NLR) compared with non-Black patients. This may suggest that the immune response in pancreatic adenocarcinoma is not what is driving disparately poor outcomes in Black patients. Further study is warranted to ascertain the role of immune response in pancreatic adenocarcinoma, the prognostic use of these measurements at diagnosis, and possible other factors, such as genetics, which may better explain poorer outcomes in Black patients.



Similar content being viewed by others
References
Oberstein PE, Olive KP. Pancreatic cancer: why is it so hard to treat? Ther Adv Gastroenterol. 2013;6(4):321–37. https://doi.org/10.1177/1756283X13478680.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Ashktorab H, Kupfer SS, Brim H, Carethers JM. Racial disparity in gastrointestinal cancer risk. Gastroenterology. 2017;153(4):910–23. https://doi.org/10.1053/j.gastro.2017.08.018.
Khawja SN, Mohammed S, Silberfein EJ, Musher BL, Fisher WE, Van Buren G. Pancreatic cancer disparities in African Americans. Pancreas. 2015;44(4):522–7. https://doi.org/10.1097/MPA.0000000000000323.
Tehranifar P, Neugut AI, Phelan JC, Link BG, Liao Y, Desai M, et al. Medical advances and racial/ethnic disparities in cancer survival. Cancer Epidemiol Biomark Prev. 2009;18(10):2701–8. https://doi.org/10.1158/1055-9965.EPI-09-0305.
Arnold LD, et al. Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity? Cancer Epidemiol Biomark Prev. 2009;18(9):2397–405. https://doi.org/10.1158/1055-9965.EPI-09-0080.
Fuchs CS, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156(19):2255–60.
Ji BT, et al. Cigarette smoking and alcohol consumption and the risk of pancreatic cancer: a case-control study in Shanghai, China. Cancer Causes Control. 1995;6(4):369–76.
Friedman GD, van den Eeden SK. Risk factors for pancreatic cancer: an exploratory study. Int J Epidemiol. 993;22(1):30–7.
Shibata A, Mack TM, Paganini-Hill A, Ross RK, Henderson BE. A prospective study of pancreatic cancer in the elderly. Int J Cancer. 1994;58(1):46–9.
Silverman DT, et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiology. 2003;14(1):45–54. https://doi.org/10.1097/01.EDE.0000034393.39604.FF.
Wong S, Gu N, Banerjee M, Birkmeyer J, Birkmeyer N. The impact of socioeconomic status on cancer care and survival. J Clin Oncol. 2011;29(15):6004–4.
M. Kogevinas and M. Porta, “Socioeconomic differences in cancer survival: a review of the evidence,” IARC Sci Publ, no. 138, pp. 177–206, 1997.
van Loon A, Brug J, Goldbohm R, van den Brandt P. Differences in cancer incidence and mortality among socio-economic groups. Scand J Soc Med. 1995;23(2):110–20.
Abraham A, Al-Refaie WB, Parsons HM, Dudeja V, Vickers SM, Habermann EB. Disparities in pancreas cancer care. Ann Surg Oncol. 2013;20(6):2078–87. https://doi.org/10.1245/s10434-012-2843-z.
Riall TS, Townsend CM, Kuo YF, Freeman JL, Goodwin JS. Dissecting racial disparities in the treatment of patients with locoregional pancreatic cancer: a 2-step process. Cancer. 2010;116(4):930–9. https://doi.org/10.1002/cncr.24836.
Singal V, Singal AK, Kuo YF. Racial disparities in treatment for pancreatic cancer and impact on survival: a population-based analysis. J Cancer Res Clin Oncol. 2012;138(4):715–22. https://doi.org/10.1007/s00432-012-1156-8.
Wray CJ, Castro-Echeverry E, Silberfein EJ, Ko TC, Kao LS. A multi-institutional study of pancreatic cancer in Harris County, Texas: race predicts treatment and survival. Ann Surg Oncol. 2012;19(9):2776–81. https://doi.org/10.1245/s10434-012-2361-z.
Heestand GM, Kurzrock R. Molecular landscape of pancreatic cancer: implications for current clinical trials. Oncotarget. 2015;6(7):4553–61. https://doi.org/10.18632/oncotarget.2972.
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. Sep 2008;321(5897):1801–6. https://doi.org/10.1126/science.1164368.
Wood LD. Pancreatic cancer genomes: toward molecular subtyping and novel approaches to diagnosis and therapy. Mol Diagn Ther. 2013;17(5):287–97. https://doi.org/10.1007/s40291-013-0043-6.
Ferri MJ, et al. Improved pancreatic adenocarcinoma diagnosis in jaundiced and non-jaundiced pancreatic adenocarcinoma patients through the combination of routine clinical markers associated to pancreatic adenocarcinoma pathophysiology. PLoS One. 2016;11(1):e0147214. https://doi.org/10.1371/journal.pone.0147214.
Inoue D, et al. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol. 2015;45(1):61–6. https://doi.org/10.1093/jjco/hyu159.
Szkandera J, Stotz M, Absenger G, Stojakovic T, Samonigg H, Kornprat P, et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer. 2014;110(1):183–8. https://doi.org/10.1038/bjc.2013.701.
Arima K, Okabe H, Hashimoto D, Chikamoto A, Tsuji A, Yamamura K, et al. The diagnostic role of the neutrophil-to-lymphocyte ratio in predicting pancreatic ductal adenocarcinoma in patients with pancreatic diseases. Int J Clin Oncol. 2016;21(5):940–5. https://doi.org/10.1007/s10147-016-0975-z.
Chen Y, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. https://doi.org/10.1186/s12885-016-2352-8.
Chen Y, Yan H, Wang Y, Shi Y, Dai G. Significance of baseline and change in neutrophil-to-lymphocyte ratio in predicting prognosis: a retrospective analysis in advanced pancreatic ductal adenocarcinoma. Sci Rep. 2017;7(1):753. https://doi.org/10.1038/s41598-017-00859-5.
Cheng H, Long F, Jaiswar M, Yang L, Wang C, Zhou Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. Sci Rep. 2015;5:11026. https://doi.org/10.1038/srep11026.
Luo G, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22(2):670–6. https://doi.org/10.1245/s10434-014-4021-y.
R. J. Hu, J. Y. Ma, and G. Hu, “Lymphocyte-to-monocyte ratio in pancreatic cancer: prognostic significance and meta-analysis,” Clin Chim Acta, vol. 481, pp. 142–146, 2018, doi: https://doi.org/10.1016/j.cca.2018.03.008.
Li W, Tao L, Zhang L, Xiu D. Prognostic role of lymphocyte to monocyte ratio for patients with pancreatic cancer: a systematic review and meta-analysis. Onco Targets Ther. 2017;10:3391–7. https://doi.org/10.2147/OTT.S142022.
Singh G, Nassri A, Kim D, Zhu H, Ramzan Z. Lymphocyte-to-monocyte ratio can predict mortality in pancreatic adenocarcinoma. World J Gastrointest Pharmacol Ther. Feb 2017;8(1):60–6. https://doi.org/10.4292/wjgpt.v8.i1.60.
Xue P, et al. Validation of lymphocyte-to-monocyte ratio as a prognostic factor in advanced pancreatic cancer: An East Asian Cohort Study of 2 Countries. Pancreas. 2017;46(8):1011–7. https://doi.org/10.1097/MPA.0000000000000891.
Ter Veer E, et al. Consensus statement on mandatory measurements in pancreatic cancer trials (COMM-PACT) for systemic treatment of unresectable disease. Lancet Oncol. 2018;19(3):e151–60. https://doi.org/10.1016/S1470-2045(18)30098-6.
Alagappan M, et al. Albumin and neutrophil-lymphocyte ratio (NLR) predict survival in patients with pancreatic adenocarcinoma treated with SBRT. Am J Clin Oncol. 2018;41(3):242–7. https://doi.org/10.1097/COC.0000000000000263.
Suzuki R, Takagi T, Hikichi T, Konno N, Sugimoto M, Watanabe KO, et al. Derived neutrophil/lymphocyte ratio predicts gemcitabine therapy outcome in unresectable pancreatic cancer. Oncol Lett. 2016;11(5):3441–5. https://doi.org/10.3892/ol.2016.4381.
Szkandera J, et al. External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PLoS One. 2013;8(11):e78225. https://doi.org/10.1371/journal.pone.0078225.
Tsujita E, et al. Postoperative neutrophil-to-lymphocyte ratio as a predictor of long-term prognosis after pancreatectomy for pancreatic carcinoma: a retrospective analysis. Am Surg. 2017;83(6):610–6.
Nemer L, et al. Predictors of pancreatic cancer-associated weight loss and nutritional interventions. Pancreas. 2017;46(9):1152–7. https://doi.org/10.1097/MPA.0000000000000898.
Yazdanbakhsh K, et al. Molecular mechanisms that lead to reduced expression of duffy antigens. Transfusion. 2000;40(3):310–20. https://doi.org/10.1046/j.1537-2995.2000.40030310.x.
Reich D, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. 2009;5(1):e1000360. https://doi.org/10.1371/journal.pgen.1000360.
Schnabel RB, Baumert J, Barbalic M, Dupuis J, Ellinor PT, Durda P, et al. Duffy antigen receptor for chemokines (Darc) polymorphism regulates circulating concentrations of monocyte chemoattractant protein-1 and other inflammatory mediators. Blood. 2010;115(26):5289–99. https://doi.org/10.1182/blood-2009-05-221382.
Wang J, et al. Enhanced expression of Duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potential. Oncogene. 2006;25(54):7201–11. https://doi.org/10.1038/sj.onc.1209703.
Zijlstra A, Quigley JP. The DARC side of metastasis: shining a light on KAI1-mediated metastasis suppression in the vascular tunnel. Cancer Cell. 2006;10(3):177–8. https://doi.org/10.1016/j.ccr.2006.08.012.
Acknowledgments
The authors would like to thank Lance K. Heilbrun, Ph.D. for his critical statistical advice.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Benjamin E. Ueberroth, Adnan Khan, and Kevin Zhang. The first draft of the manuscript was written by Benjamin E. Ueberroth and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ueberroth, B.E., Khan, A., Zhang, K.J. et al. Differences in Baseline Characteristics and White Blood Cell Ratios Between Racial Groups in Patients with Pancreatic Adenocarcinoma. J Gastrointest Canc 52, 160–168 (2021). https://doi.org/10.1007/s12029-020-00378-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-020-00378-z