Abstract
Purpose
Despite new treatment options in metastatic colorectal cancer (mCRC), new prognostic markers are still needed to determine optimal chemoregimen especially for anti-angiogenesis drugs. In this study, we evaluated the serum semaphorin and VEGF-A levels as prognostic factors in patients with mCRC.
Methods
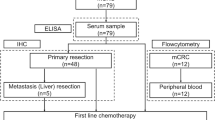
Patients with diagnosed mCRC who were treated with first-line bevacizumab plus chemotherapy were included in the study. Venous blood samples of 37 patients with metastatic colon cancer were taken, and serum semaphorin 3A and VEGF-A levels were studied in pre-treatment and the 1st and third months after the treatment was initiated.
Results
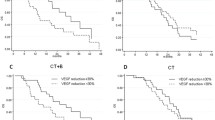
Totally, 37 patients were enrolled in the study. The patients’ mean age was 62 years. Twenty-eight (49%) of the patients were male, and 19 (51%) were female. Serum semaphorin3A (sema3A) levels of the patients were 5.4 ± 7.4 ng/ml before the treatment, 3.5 ± 3.3 ng/ml at the first month, and 3.5 ± 3.7 ng/ml at the third month. Serum VEGF-A levels were 27.7 ± 32.9 ng/l before the treatment, 23.1 ± 28.1 ng/l at the first month, and 28.9 ± 30.2 ng/l at the third month. There was no significant correlation between the survival and pre-treatment VEGF-A level (p = 0.064). Overall survival (OS) was statistically significantly higher in patients with pre-treatment semaphorin 3A levels below 5.4 ng/ml than higher than 5.4 ng/ml (10.5 months vs 4.5 months, respectively, HR 0.23, 95% CI 19.635–11,391, p = 0.012).
Conclusion
Pre-treatment semaphorin 3A level can be a prognostic marker for the mCRC patients who were treated with bevacizumab in patients with metastatic colorectal cancer.

Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. https://doi.org/10.3322/caac.21262.
Karaman S, Leppanen VM, Alitalo K. Vascular endothelial growth factor signaling in development and disease. Development. 2018;145:dev151019. https://doi.org/10.1242/dev.151019.
Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–25. https://doi.org/10.1038/nrm.2016.87.
Kramer I, Lipp HP. Bevacizumab, a humanized anti-angiogenic monoclonal antibody for the treatment of colorectal cancer. J Clin Pharm Ther. 2007;32:1–14. https://doi.org/10.1111/j.1365-2710.2007.00800.x.
Hurwitz H, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–8. https://doi.org/10.1200/JCO.2005.10.017.
Heinemann V, Von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75. https://doi.org/10.1016/S1470-2045(14)70330-4.
Heinemann V, Rivera F, O’Neil BH, et al. A study-level meta-analysis of efficacy data from head-to-head first-line trials of epidermal growth factor receptor inhibitors versus bevacizumab in patients with RAS wild-type metastatic colorectal cancer. Eur J Cancer. 2016;67:11–20. https://doi.org/10.1016/j.ejca.2016.07.019.
Passardi A, Nanni O, Tassinari D, Turci D, Cavanna L, Fontana A, et al. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: final results for first-line treatment from the ITACa randomized clinical trial. Ann Oncol. 2015;26:1201–7. https://doi.org/10.1093/annonc/mdv130.
Hei Yuan HS, Katyal S, Anderson JE. A mechanism for semaphorin-induced apoptosis: DNA damage of endothelial and myogenic cells in primary cultures from skeletal muscle. Oncotarget. 2018;9:22618–30. https://doi.org/10.18632/oncotarget.25200.
Sumi C, Hirose N, Yanoshita M, Takano M, Nishiyama S, Okamoto Y, et al. Semaphorin 3A inhibits inflammation in chondrocytes under excessive mechanical stress. Mediat Inflamm. 2018;2018:5703651. https://doi.org/10.1155/2018/5703651.
Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–9. https://doi.org/10.1038/nm1505.
Lepelletier Y, Moura IC, Hadj-Slimane R, et al. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol. 2006;36:1782–93. https://doi.org/10.1002/eji.200535601.
Gaur P, Bielenberg DR, Samuel S, Bose D, Zhou Y, Gray MJ, et al. Role of class 3 semaphorins and their receptors in tumor growth and angiogenesis. Clin Cancer Res. 2009;15:6763–70. https://doi.org/10.1158/1078-0432.CCR-09-1810.
Staton CA. Class 3 semaphorins and their receptors in physiological and pathological angiogenesis. Biochem Soc Trans. 2011;39:1565–70. https://doi.org/10.1042/BST20110654.
Maione F, Molla F, Meda C, Latini R, Zentilin L, Giacca M, et al. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J Clin Invest. 2009;119:3356–72. https://doi.org/10.1172/JCI36308.
Groppa E, Brkic S, Bovo E, Reginato S, Sacchi V, di Maggio N, et al. VEGF dose regulates vascular stabilization through semaphorin3A and the neuropilin-1+ monocyte/TGF-beta1 paracrine axis. EMBO molecular medicine. 2015;7:1366–84. https://doi.org/10.15252/emmm.201405003.
Takamatsu H, Okuno T, Kumanogoh A. Regulation of immune cell responses by semaphorins and their receptors. Cellular & molecular immunology. 2010;7:83–8. https://doi.org/10.1038/cmi.2009.111.
Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, et al. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–3.
Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int J Oncol. 2007;30:1231–8.
Neufeld G, Sabag AD, Rabinovicz N, Kessler O. Semaphorins in angiogenesis and tumor progression. Cold Spring Harbor perspectives in medicine. 2012;2:a006718. https://doi.org/10.1101/cshperspect.a006718.
Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995;14:949–59.
Acevedo LM, Barillas S, Weis SM, Gothert JR, Cheresh DA. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood. 2008;111:2674–80. https://doi.org/10.1182/blood-2007-08-110205.
Maione F, Capano S, Regano D, Zentilin L, Giacca M, Casanovas O, et al. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J Clin Invest. 2012;122:1832–48. https://doi.org/10.1172/JCI58976.
Chakraborty G, Kumar S, Mishra R, Patil TV, Kundu GC. Semaphorin 3A suppresses tumor growth and metastasis in mice melanoma model. PLoS One. 2012;7:e33633. https://doi.org/10.1371/journal.pone.0033633.
Casazza A, Fu X, Johansson I, Capparuccia L, Andersson F, Giustacchini A, et al. Systemic and targeted delivery of semaphorin 3A inhibits tumor angiogenesis and progression in mouse tumor models. Arterioscler Thromb Vasc Biol. 2011;31:741–9. https://doi.org/10.1161/ATVBAHA.110.211920.
Lavi N, Kessler O, Ziv K, Nir-Zvi I, Mumblat Y, Eiza N, et al. Semaphorin-3A inhibits multiple myeloma progression in a mouse model. Carcinogenesis. 2018;39(10):1283–91. https://doi.org/10.1093/carcin/bgy106.
Lee J, Shin YJ, Lee K, Cho HJ, Sa JK, Lee SY. Anti-SEMA3A antibody: a novel therapeutic agent to suppress GBM tumor growth. Cancer Res Treat. 2018;50(3):1009–22. https://doi.org/10.4143/crt.2017.315.
Catalano A, Caprari P, Moretti S, Faronato M, Tamagnone L, Procopio A. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood. 2006;107(8):3321–9. https://doi.org/10.1182/blood-2005-06-2445.
Liu F, Shen W, Qiu H, Hu X, Zhang C, Chu T. Prostate cancer cells induce osteoblastic differentiation via semaphorin 3A. Prostate. 2015;75:370–80. https://doi.org/10.1002/pros.22923.
Hu ZQ, Zhou SL, Zhou ZJ, Luo CB, Chen EB, Zhan H, et al. Overexpression of semaphorin 3A promotes tumor progression and predicts poor prognosis in hepatocellular carcinoma after curative resection. Oncotarget. 2016;7:51733–46. https://doi.org/10.18632/oncotarget.10104.
Li X, Chen Q, Yin D, Shi S, Yu L, Zhou S, et al. Novel role of semaphorin 3A in the growth and progression of hepatocellular carcinoma. Oncol Rep. 2017;37:3313–20. https://doi.org/10.3892/or.2017.5616.
Tang C, Gao X, Liu H, Jiang T, Zhai X. Decreased expression of SEMA3A is associated with poor prognosis in gastric carcinoma. Int J Clin Exp Pathol. 2014;7:4782–94.
Vadasz Z, Rubinstein J, Bajer J, Sheffer H, Halachmi S. Overexpression of semaphorin 3A in patients with urothelial cancer. Urol Oncol. 2018;36(4):161.e1–6. https://doi.org/10.1016/j.urolonc.2017.12.007.
Müller M, Giese N, Swiercz J, Ceyhan G, Esposito I, Hinz U, et al. Association of axon guidance factor Semaphorin 3A with poor outcome in pancreatic cancer. Int J Cancer. 2007;121:2421–33. https://doi.org/10.1002/ijc.22949.
Basile J, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25:6889–98. https://doi.org/10.1128/MCB.25.16.6889-6898.2005.
Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, et al. Semaphorin-4A, an activator for T-cell -mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J. 2007;26:1373–84. https://doi.org/10.1038/sj.emboj.7601589.
Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–30. https://doi.org/10.1016/j.humimm.2009.02.008.
Tsai HL, Lin CH, Huang CW, et al. Decreased peritherapeutic VEGF expression could be a predictor of responsiveness to first-line FOLFIRI plus bevacizumab in mCRC patients. Int J Clin Exp Pathol. 2015;8:1900–10.
Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, et al. Impact of vascular endothelial growth factor-a expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217–27. https://doi.org/10.1200/JCO.2005.01.5388.
Hoff PM, Hochhaus A, Pestalozzi BC, Tebbutt NC, Li J, Kim TW, et al. Cediranib plus FOLFOX/CAPOX versus placebo plus FOLFOX/CAPOX in patients with previously untreated metastatic colorectal cancer: a randomized, double-blind, phase III study (HORIZON II). J Clin Oncol. 2012;30(29):3596–603. https://doi.org/10.1200/JCO.2012.42.6031.
Kara O, Duman BB, Kara B, Erdogan S, Parsak CK, Sakman G. Analysis of PTEN, VEGF, HER2 and P53 status in determining colorectal cancer benefit from bevacizumab therapy. Asian Pac J Cancer Prev. 2012;13(12):6397–401.
Dowlati A, Gray R, Sandler AB, Schiller JH, Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab–an Eastern Cooperative Oncology Group study. Clin Cancer Res. 2008;14(5):1407–12. https://doi.org/10.1158/1078-0432.CCR-07-1154.
Acknowledgments
We thank Necmettin Erbakan University for supporting this trial, and I thank the all other authors for providing language help, writing assistance, and proofreading the article.
Funding
This work was supported by Necmettin Erbakan University Scientific Research Project Committee with a project number 151518014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The ethical approval was obtained from Necmettin Erbakan University Meram Faculty of Medicine, Ethics Committee of the Non-Medical Devices Research (decision number 2015-169), before the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karpuz, T., Araz, M., Korkmaz, L. et al. The Prognostic Value of Serum Semaphorin3A and VEGF Levels in Patients with Metastatic Colorectal Cancer. J Gastrointest Canc 51, 491–497 (2020). https://doi.org/10.1007/s12029-019-00263-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-019-00263-4