Abstract
Background
Although endoscopic resection is increasingly performed to treat submucosal invasive colorectal cancer (T1CRC), approximately 10% are at risk of lymph node metastasis. The Japanese Society for Cancer of the Colon and Rectum guideline indicates that the following risk factors should be considered when deciding whether to perform additional surgical resection with lymph node dissection: depth of T1 invasion, lymphovascular invasion, poor histological grade, and budding grade 2/3. However, there is little information about the prognosis of T1CRC patients, or factors to consider when deciding subsequent treatment of high-risk T1CRC.
Methods
This retrospective mixed method study was conducted using electronic medical records at Kyoto University Hospital between February 2005 and February 2015. Participants were T1CRC patients at risk of lymph node metastasis with at least one of the above four risk factors. They were assigned either careful follow-up (FU) or additional surgery (AS) through shared decision-making. To identify factors affecting decision-making in the FU group, we performed qualitative content analysis of electronic medical records. The prognosis of the groups was compared using the Kaplan–Meier method and the log-rank test.
Results
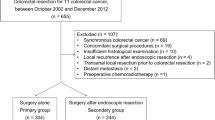
Of 161 T1CRC patients, 18 were included in the FU group and 19 in the AS group. The median follow-up time was 39.5 (range 23–126) months for the FU group and 62 (range 22–141) months for the AS group. Factors considered in selecting FU were advanced age, comorbidities, the sole presence of the “depth” risk factor, and lower rectal cancer. For AS, the risk factors cited in the guideline were considered. There was one recurrent case in each group during the research period. There were no significant differences in overall survival, cause-specific survival, or recurrence-free survival between the groups.
Conclusions
Age, comorbidities, and lower-rectal cancer location were considered in deciding posttreatment strategy among high-risk T1CRC patients, alongside with positive vertical margin, depth, lymphovascular invasion, poor histologic grade, and budding. During the research period, there was no prognostic difference between the FU and AS groups.


Similar content being viewed by others
References
Ferlay J, Soerjomataram II, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2014;136:E359–86. https://doi.org/10.1002/ijc.29210.
National Cancer Center [Cancer information service] Gan joho service (in Japanese). http://ganjoho.jp/reg_stat/statistics/stat/summary.html. Accessed 5 Feb 2018.
Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502. https://doi.org/10.1016/S0140-6736(13)61649-9.
Read TE, Mutch MG, Chang BW, McNevin MS, Fleshman JW, Birnbaum EH, et al. Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J Am Coll Surg. 2002;195:33–40. https://doi.org/10.1016/S1072-7515(02)01224-3.
Kobayashi H, Mochizuki H, Morita T, Kotake K, Teramoto T, Kameoka S, et al. Characteristics of recurrence after curative resection for T1 colorectal cancer: Japanese multicenter study. J Gastroenterol. 2011;46:203–11. https://doi.org/10.1007/s00535-010-0341-2.
Tanaka S, Asayama N, Shigita K, Hayashi N, Oka S, Chayama K. Towards safer and appropriate application of endoscopic submucosal dissection for T1 colorectal carcinoma as total excisional biopsy: future perspectives. Dig Endosc. 2015;27:216–22. https://doi.org/10.1111/den.12326.
Tanaka S, Yokota T, Saito D, Okamoto S, Oguro Y, Yoshida S. Clinicopathologic features of early rectal carcinoma and indications for endoscopic treatment. Dis Colon Rectum. 1995;38:959–63.
Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551–9. https://doi.org/10.1053/j.gastro.2012.12.003.
Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2017;23:1–34. https://doi.org/10.1007/s10147-017-1101-6.
Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;127:93–9. https://doi.org/10.1097/SLA.0b013e3181b45598.
Hilditch WG, Asbury AJ, Jack E, McGrane S. Validation of a pre-anaesthetic screening questionnaire. Anaesthesia. 2003;58:874–7. https://doi.org/10.1046/j.1365-2044.2003.03341.x.
Turner NJ, Haward RA, Mulley GP, Selby PJ. Cancer in old age---is it inadequately investigated and treated? BMJ. 1999;319:309–12. https://doi.org/10.1136/bmj.319.7205.309.
Nesbakken A, Nygaard K, Bull-Njaa T, Carlsen E, Eri LM. Bladder and sexual dysfunction after mesorectal excision for rectal cancer. Br J Surg. 2000;87:206–10. https://doi.org/10.1046/j.1365-2168.2000.01357.x.
Hendren SK, O’Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg. 2005;242:212–23. https://doi.org/10.1097/01.sla.0000171299.43954.ce.
Vironen JH, Kairaluoma M, Aalto A-M, Kellokumpu IH. Impact of functional results on quality of life after rectal cancer surgery. Dis Colon Rectum. 2006;49:568–78. https://doi.org/10.1007/s10350-006-0513-6.
Barry MJ, Edgman-Levitan S. Shared decision making — the pinnacle of patient-centered care. N Engl J Med. 2012;366:780–1. https://doi.org/10.1056/NEJMp1109283.
Creswell JW, Clark VLP. Designing and conducting mixed methods research. 3rd ed. SAGE Publications; 2018.
Johnson RB, Onwuegbuzie AJ. Mixed methods research: a research paradigm whose time has come. Educ Res. 2004;33:14–26. https://doi.org/10.3102/0013189X033007014.
Lambert R, Lightdale CJ. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon. Gastrointest Endosc. 2003;58:S3–43. https://doi.org/10.1016/S0016-5107(03)02168-0.
Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2005 for the treatment of colorectal cancer (in Japanese). Kanehara & Co., Ltd; 2005.
Ueno H, Hidetaka M, Hashiguchi Y, Shimazaki H, Shinsuke A, Kazuo H, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–94. https://doi.org/10.1053/S0016-5085(04)00717-6.
Krippendorff K. Content analysis: an introduction to its methodology. 3rd ed. SAGE Publications; 2013.
Downe-Wamboldt B. Content analysis: method, applications, and issues. Health Care Women Int. 1992;13:313–21. https://doi.org/10.1080/07399339209516006.
Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–88. https://doi.org/10.1177/1049732305276687.
Fusch PI, Ness LR. Are we there yet? Data saturation in qualitative research. Qual Rep. 2015;20:1408–16.
Gamer M, Lemon J, Fellows I, Singh P. Package “ irr .” 2015. https://cran.r-project.org/web/packages/irr/irr.pdf. Accessed 5 Feb 2018.
Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–60. https://doi.org/10.1002/bjs.1800780327.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. https://doi.org/10.1016/0021-9681(87)90171-8.
Yamano T, Yamauchi S, Kimura K, Babaya A, Hamanaka M, Kobayashi M, et al. Influence of age and comorbidity on prognosis and application of adjuvant chemotherapy in elderly Japanese patients with colorectal cancer: a retrospective multicentre study. Eur J Cancer. 2017;81:90–101. https://doi.org/10.1016/j.ejca.2017.05.024.
Ajioka Y, Okura Y, Ikegami M, Ichihara M, Egashira Y, Al E. Stratification of the risk of lymph node metastasis of T1b (SM depth of more than 1,000 μm) colorectal cancer. In: Color. Dis. NOW 2016 (in Japanese only). Nihon Medical Center; 2016. p. 63–8.
Yoshii S, Nojima M, Nosho K, Omori S, Kusumi T, Okuda H, et al. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of t1 tumors. Clin Gastroenterol Hepatol. 2014;12:292–302.e3. https://doi.org/10.1016/j.cgh.2013.08.008.
Yamano T, Yamauchi S, Tsukamoto K, Noda M, Kobayashi M, Hamanaka M, et al. Evaluation of appropriate follow-up after curative surgery for patients with colorectal cancer using time to recurrence and survival after recurrence: a retrospective multicenter study. Oncotarget. 2018;9:25474–90. https://doi.org/10.18632/oncotarget.25312.
Acknowledgments
The authors express sincere gratitude to all the medical staffs involved in these cases
Funding
This study was partially supported by Kyoto Preventive Medical Center.
Author information
Authors and Affiliations
Contributions
Conception and design (YN, TH, and TN); data collection and management (YN, TH, DN, AY, MY, KH, KS, SM, HS, and YS); quantitative data analysis (YN, TH, and TN); qualitative data analysis (YN, TH, DN, and AK); interpretation of the results (all authors); drafting of the article (YN); critical revision of the article for important intellectual content (all authors); final approval of the article (all authors).
Corresponding author
Ethics declarations
This study was approved by the Ethics Committee of the Kyoto University Graduate School of Medicine.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishikawa, Y., Horimatsu, T., Nishizaki, D. et al. Qualitative and Quantitative Analysis of Posttreatment Strategy After Endoscopic Resection for Patients with T1 Colorectal Cancer at High Risk of Lymph Node Metastasis. J Gastrointest Canc 51, 242–249 (2020). https://doi.org/10.1007/s12029-019-00247-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-019-00247-4