Abstract
Purpose
Pancreatic adenocarcinoma is an untreatable cancer with a 5-year survival rate of about 6 % or less for the past 35 years. This lack of significant progress is largely due to the lack of elucidation and understanding of the factors involved in the development of this cancer. Recent studies identified and implicated zinc in the development and progression of pancreatic cancer. This study was conducted to establish the changes in zinc, ZIP3 zinc transporter, and Ras-responsive element-binding protein 1 (RREB-1) transcription factor as early events in the development of malignancy.
Methods
In situ relative zinc determination and immunohistochemical analysis of ZIP3 and RREB-1 were performed on archived human pancreatic tissue sections and tissue microarrays. Normal/benign versus adenocarcinoma pancreas was compared. Panc1 cells were employed to determine the influence of RREB-1 on ZIP3 expression.
Results
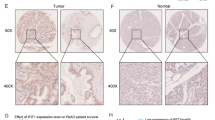
Zinc levels of normal ductal and acinar epithelium were markedly and consistently decreased in adenocarcinoma. Pancreatic intraepithelial neoplasia (PanIN) lesions also exhibited a loss of zinc. ZIP3 and RREB-1 were also markedly downregulated. Initial results indicate that RREB-1 regulates ZIP3 expression.
Conclusions
These results corroborate the earlier report that zinc, ZIP3, and RREB-1 are markedly decreased in early stage adenocarcinoma. Additionally and most importantly, these changes occur in PanIN, which are thought to be precancerous lesions leading to ductal adenocarcinoma. These results support a concept that downregulation of RREB-1 causes downregulation of ZIP3, which results in decreased zinc in premalignant and carcinoma cells. The decrease in zinc is essential to remove its cytotoxic effects on malignant cells. This relationship constitutes a new concept of early genetic/metabolic events in the progressive transformation of normal cells to premalignant cells to malignant cells in the development of pancreatic cancer.













Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Costello LC, Levy B, Desouki M, Zou J, Bagasra O, Johnson L, Hanna N, Franklin RB. Decreased zinc and down regulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Canc Biol Ther. 2011;12:297–303.
Scarlett CJ, Salisbury EL, Biankin AV, Kench J. Precursor lesions in pancreatic cancer: morphological and molecular pathology. Pathol. 2011;43:183–200.
Liszka L, Pająk J, Mrowiec S, Zielińska-Pająk E, Gołka D, Lampe P. Precursor lesions of early onset pancreatic cancer. Virchows Arch. 2011;4:439–51.
Recavarren C, Labow DM, Liang J, Zhang L, Wong M, Zhu H, Wang J, Francis F, Xu R. Histologic characteristics of pancreatic intraepithelial neoplasia associated with different pancreatic lesions. Hum Pathol. 2011;42:18–24.
Danielsen A, Steinnes E. A study of some selected trace elements in normal and cancerous tissue by neutron activation analysis. J Nucl Med. 1970;11:260–4.
Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A. 2007;104:18636–41.
Thiagalingam A, De Bustros A, Borges M, Jasti R, Compton D, Diamond L, Mabry M, Ball DW, Baylin SB, Nelkin BD. RREB-1, a novel zinc finger protein, is involved in the differentiation response to Ras in human medullary thyroid carcinomas. Mol Cell Biol. 1996;16:5335–45.
Fujimoto-Nishiyama A, Ishii S, Matsuda S, Inoue J, Yamamoto T. A novel zinc finger protein, Finb, is a transcriptional activator and localized in nuclear bodies. Gene. 1997;195:267–75.
Liu H, Hew HC, Lu ZG, Yamaguchi T, Miki Y, Yoshida K. DNA damage signaling recruits RREB-1 to the p53 tumour suppressor promoter. Biochem J. 2009;422:543–51.
Jiang W, Sequeira JM, Nakayama Y, Lai SC, Quadros EV. Characterization of the promoter region of TCblR/CD320 gene, the receptor for cellular uptake of transcobalamin-bound cobalamin. Gene. 2010;15:49–55.
Mukhopadhyay NK, Cinar B, Mukhopadhyay L, Lutchman M, Ferdinand AS, Kim J, Chung LW, Adam RM, Ray SK, Leiter AB, Richie JP, Liu BC, Freeman MR. The zinc finger protein ras-responsive element binding protein-1 is a coregulator of the androgen receptor: implications for the role of the Ras pathway in enhancing androgenic signaling in prostate cancer. Mol Endocrinol. 2007;21:2056–70.
Flajollet S, Poras I, Carosella ED, Moreau P. RREB-1 is a transcriptional repressor of HLA-G. J Immunol. 2009;183:6948–59.
Milon BC, Agyapong A, Bautista R, Costello LC, Franklin RB. (2010) Ras responsive element binding protein-1 (RREB-1) down-regulates hZIP1 expression in prostate cancer cells. Prostate. 2010;70:118–29.
Zou J, Milon BC, Desouki MM, Costello LC, Franklin RB. hZIP1 zinc transporter down-regulation in prostate cancer involves the overexpression of ras responsive element binding protein-1 (RREB-1). Prostate. 2012. doi:10.1002/pros.21368.
Oxford G, Smith SC, Hampton G, Theodorescu D. Expression profiling of Ral-depleted bladder cancer cells identifies RREB-1 as a novel transcriptional Ral effector. Oncogene. 2007;26:7143–752.
Jayaraman AK, Jayaraman S. Increased level of exogenous zinc induces cytotoxicity and up-regulates the expression of the ZnT-1 zinc transporter gene in pancreatic cancer cells. J Nutr Biochem. 2011;22:79–88.
Donadelli M, Dalla Pozza E, Scupoli MT, Costanzo C, Scarpa A, Palmieri M. Intracellular zinc increase inhibits p53(−/−) pancreatic adenocarcinoma cell growth by ROS/AIF-mediated apoptosis. Biochim Biophys Acta. 2009;1793:273–80.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costello, L.C., Zou, J., Desouki, M.M. et al. Evidence for Changes in RREB-1, ZIP3, and Zinc in the Early Development of Pancreatic Adenocarcinoma. J Gastrointest Canc 43, 570–578 (2012). https://doi.org/10.1007/s12029-012-9378-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-012-9378-1