Abstract
Background
The Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase II randomized controlled trial used a tier-based management protocol based on brain tissue oxygen (PbtO2) and intracranial pressure (ICP) monitoring to reduce brain tissue hypoxia after severe traumatic brain injury. We performed a secondary analysis to explore the relationship between brain tissue hypoxia, blood pressure (BP), and interventions to improve cerebral perfusion pressure (CPP). We hypothesized that BP management below the lower limit of autoregulation would lead to cerebral hypoperfusion and brain tissue hypoxia that could be improved with hemodynamic augmentation.
Methods
Of the 119 patients enrolled in the Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase II trial, 55 patients had simultaneous recordings of arterial BP, ICP, and PbtO2. Autoregulatory function was measured by interrogating changes in ICP and PbtO2 in response to fluctuations in CPP using time-correlation analysis. The resulting autoregulatory indices (pressure reactivity index and oxygen reactivity index) were used to identify the “optimal” CPP and limits of autoregulation for each patient. Autoregulatory function and percent time with CPP outside personalized limits of autoregulation were calculated before, during, and after all interventions directed to optimize CPP.
Results
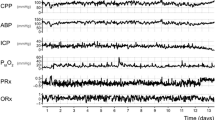
Individualized limits of autoregulation were computed in 55 patients (mean age 38 years, mean monitoring time 92 h). We identified 35 episodes of brain tissue hypoxia (PbtO2 < 20 mm Hg) treated with CPP augmentation. Following each intervention, mean CPP increased from 73 ± 14 mm Hg to 79 ± 17 mm Hg (p = 0.15), and mean PbtO2 improved from 18.4 ± 5.6 mm Hg to 21.9 ± 5.6 mm Hg (p = 0.01), whereas autoregulatory function trended toward improvement (oxygen reactivity index 0.42 vs. 0.37, p = 0.14; pressure reactivity index 0.25 vs. 0.21, p = 0.2). Although optimal CPP and limits remained relatively unchanged, there was a significant decrease in the percent time with CPP below the lower limit of autoregulation in the 60 min after compared with before an intervention (11% vs. 23%, p = 0.05).
Conclusions
Our analysis suggests that brain tissue hypoxia is associated with cerebral hypoperfusion characterized by increased time with CPP below the lower limit of autoregulation. Interventions to increase CPP appear to improve autoregulation. Further studies are needed to validate the importance of autoregulation as a modifiable variable with the potential to improve outcomes.



Similar content being viewed by others
References
Wang A, Ortega-Gutierrez S, Petersen NH. Autoregulation in the neuro ICU. Curr Treat Option Ne. 2018;20:20.
Czosnyka M, Miller C. Monitoring P in the IMCC on M. Monitoring of cerebral autoregulation. Neurocrit Care. 2014;21(Suppl 2):S95-102.
Roux PL, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, et al. Consensus summary statement of the international multidisciplinary consensus conference on multimodality monitoring in neurocritical care: a statement for healthcare professionals from the neurocritical care society and the European society of intensive care medicine. Neurocrit Care. 2014;21(Suppl 2):S1-26.
Aries MJH, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–63.
Donnelly J, Czosnyka M, Adams H, Robba C, Steiner LA, Cardim D, et al. Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med. 2017;45:1464–71.
Okonkwo DO, Shutter LA, Moore C, Temkin NR, Puccio AM, Madden CJ, et al. Brain oxygen optimization in severe traumatic brain injury phase-II. Crit Care Med. 2017;45:1907–14.
Beqiri E, Smielewski P, Robba C, Czosnyka M, Cabeleira MT, Tas J, et al. Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open. 2019;9:e030727.
Liu X, Maurits NM, Aries MJH, Czosnyka M, Ercole A, Donnelly J, et al. Monitoring of optimal cerebral perfusion pressure in traumatic brain injured patients using a multi-window weighting algorithm. J Neurotraum. 2017;34:3081–8.
Depreitere B, Güiza F, den Berghe GV, Schuhmann MU, Maier G, Piper I, et al. Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. J Neurosurg. 2014;120:1451–7.
Bagiella E, Novack TA, Ansel B, Diaz-Arrastia R, Dikmen S, Hart T, et al. Measuring outcome in traumatic brain injury treatment trials. J Head Trauma Rehab. 2010;25:375–82.
Marín-Caballos AJ, Murillo-Cabezas F, Cayuela-Domínguez A, Domínguez-Roldán JM, Rincón-Ferrari MD, Valencia-Anguita J, et al. Cerebral perfusion pressure and risk of brain hypoxia in severe head injury: a prospective observational study. Crit Care. 2005;9:R670.
Schmidt JM, Ko S-B, Helbok R, Kurtz P, Stuart RM, Presciutti M, et al. Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke. 2011;42:1351–6.
Megjhani M, Weiss M, Ford J, Terilli K, Kastenholz N, Nametz D, et al. Optimal cerebral perfusion pressure and brain tissue oxygen in aneurysmal subarachnoid hemorrhage. Stroke. 2022.
Chang JJJ, Youn TS, Benson D, Mattick H, Andrade N, Harper CR, et al. Physiologic and functional outcome correlates of brain tissue hypoxia in traumatic brain injury. Crit Care Med. 2009;37:283–90.
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury fourth edition. Neurosurgery. 2017;80:6–15.
Chesnut R, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020;46:919–29.
Hawryluk GWJ, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019;45:1783–94.
Aries MJH, Wesselink R, Elting JWJ, Donnelly J, Czosnyka M, Ercole A, et al. Enhanced visualization of optimal cerebral perfusion pressure over time to support clinical decision making. Crit Care Med. 2016;44:e996–9.
Silverman A, Kodali S, Strander S, Gilmore EJ, Kimmel A, Wang A, et al. Deviation from personalized blood pressure targets is associated with worse outcome after subarachnoid hemorrhage. Stroke. 2019;50:2729–37.
Petersen NH, Silverman A, Strander SM, Kodali S, Wang A, Sansing LH, et al. Fixed compared with autoregulation-oriented blood pressure thresholds after mechanical thrombectomy for ischemic stroke. Stroke. 2020;51:914–21.
Tas J, Beqiri E, van Kaam RC, Czosnyka M, Donnelly J, Haeren RH, et al. Targeting autoregulation-guided cerebral perfusion pressure after traumatic brain injury (COGiTATE): a feasibility randomized controlled clinical trial. J Neurotraum. 2021;38:2790–800.
Sorrentino E, Diedler J, Kasprowicz M, Budohoski KP, Haubrich C, Smielewski P, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care. 2012;16:258–66.
Czosnyka M, Czosnyka Z, Smielewski P. Pressure reactivity index: journey through the past 20 years. Acta Neurochir. 2017;159:2063–5.
Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity&ast. Crit Care Med. 2006;34:1783–8.
Owen B, Vangala A, Fritch C, Alsarah AA, Jones T, Davis H, et al. Cerebral autoregulation correlation with outcomes and spreading depolarization in aneurysmal subarachnoid hemorrhage. Stroke. 2022;53:1975–83.
Author information
Authors and Affiliations
Contributions
AP, AdH, and NHP contributed to the study concept and design. NRT, JB, and RDA contributed to data acquisition. AP, EJG, SOG, AdH, and NHP contributed to the analyses and interpretation of data. AP, EJG, JAK, LB, MO, RB, GJF, CM, SOG, NRT, JB, RDA, AdH, and NHP contributed to the drafting and critical revisions of the manuscript. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
NHP is supported by the National Institutes of Health - National Institute of Neurological Disorders and Stroke (NIH-NINDS) (K23NS110980) and has received clinical research funding from Liminal Sciences. ADH is supported by NIH-NINDS (K23NS105924), has received investigator-initiated clinical research funding from the AAN, has received consultant fees from Integra and Novo Nordisk, has equity in TitinKM and Certus, and receives author fees from UpToDate. EJG is supported by NIH-NINDS (R01NS117904) and receives consulting fees from UCB. SOG is supported by NIH-NINDS (R01NS127114, R03NS1202228), industry investigator-initiated grants from Stryker Neurovascular, Medtronic, Microvention, Methinks, and VizAi, and Society of Vascular and Interventional Neurology, and consulting fees from Medtronic, Stryker, and Microvention. CCM is supported by NIH-NINDS R21NS119992, receives consulting fees from Microvention-Terumo and Stryker, and speaker fees from Penumbra and Silk Road Medical. NRT and JB are supported by NIH-NINDS (U01NS099084).
Ethical Approval/Informed Consent
The Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase II trial was approved by the local institutional review board at each participating center, and proxy informed consent was obtained from each study participant. This secondary analysis was performed on deidentified data obtained from the Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase II investigators under a data use agreement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prasad, A., Gilmore, E.J., Kim, J.A. et al. Impact of Therapeutic Interventions on Cerebral Autoregulatory Function Following Severe Traumatic Brain Injury: A Secondary Analysis of the BOOST-II Study. Neurocrit Care (2023). https://doi.org/10.1007/s12028-023-01896-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12028-023-01896-x