Abstract
Background
Little is known about the natural history of comatose patients with brain injury, as in many countries most of these patients die in the context of withdrawal of life-sustaining therapies (WLSTs). The accuracy of predicting recovery that is used to guide goals-of-care decisions is uncertain. We examined long-term outcomes of patients with ischemic or hemorrhagic stroke predicted by experienced clinicians to have no chance of meaningful recovery in Japan, where WLST in patients with isolated neurological disease is uncommon.
Methods
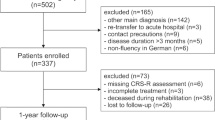
We retrospectively reviewed the medical records of all patients admitted with acute ischemic stroke, intracerebral hemorrhage, or nontraumatic subarachnoid hemorrhage between January 2018 and December 2020 to a neurocritical care unit at Toda Medical Group Asaka Medical Center in Saitama, Japan. We screened for patients who were predicted by the attending physician on postinjury day 1–4 to have no chance of meaningful recovery. Primary outcome measures were disposition at hospital discharge and the ability to follow commands and functional outcomes measured by the Glasgow Outcome Scale-Extended (GOS-E), which was assessed 6 months after injury.
Results
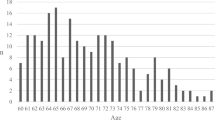
From 860 screened patients, we identified 40 patients (14 with acute ischemic stroke, 19 with intracerebral hemorrhage, and 7 with subarachnoid hemorrhage) who were predicted to have no chance of meaningful recovery. Median age was 77 years (interquartile range 64–85), 53% (n = 21) were women, and 80% (n = 32) had no functional deficits prior to hospitalization. Six months after injury, 17 patients were dead, 14 lived in a long-term care hospital, 3 lived at home, 2 lived in a rehabilitation center, and 2 lived in a nursing home. Three patients reliably followed commands, two were in a vegetative state (GOS-E 2), four fully depended on others and required constant assistance (GOS-E 3), one could be left alone independently for 8 h per day but remained dependent (GOS-E 4), and one was independent and able to return to work-like activities (GOS-E 5).
Conclusions
In the absence of WLST, almost half of the patients predicted shortly after the injury to have no chance of meaningful recovery were dead 6 months after the injury. A small minority of patients had good functional recovery, highlighting the need for more accurate neurological prognostication.


Similar content being viewed by others
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hammond FM, Katta-Charles S, Russell MB, et al. Research needs for prognostic modeling and trajectory analysis in patients with disorders of consciousness. Neurocrit Care. 2021;35(Suppl 1):55–67. https://doi.org/10.1007/s12028-021-01289-y.
Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke. 2003;34(7):1717–22. https://doi.org/10.1161/01.STR.0000078657.22835.B9.
Flint AC, Cullen SP, Faigeles BS, Rao VA. Predicting long-term outcome after endovascular stroke treatment: the totaled health risks in vascular events score. AJNR Am J Neuroradiol. 2010;31(7):1192–6. https://doi.org/10.3174/ajnr.A2050.
Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891–7. https://doi.org/10.1161/01.str.32.4.891.
Myint PK, Clark AB, Kwok CS, et al. The SOAR (stroke subtype, oxford community stroke project classification, age, prestroke modified Rankin) score strongly predicts early outcomes in acute stroke. Int J Stroke. 2014;9(3):278–83. https://doi.org/10.1111/ijs.12088.
Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke. 2008;39(8):2304–9. https://doi.org/10.1161/STROKEAHA.107.512202.
Ruiz-Sandoval JL, Chiquete E, Romero-Vargas S, Padilla-Martinez JJ, Gonzalez-Cornejo S. Grading scale for prediction of outcome in primary intracerebral hemorrhages. Stroke. 2007;38(5):1641–4. https://doi.org/10.1161/STROKEAHA.106.478222.
Saposnik G, Kapral MK, Liu Y, et al. IScore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation. 2011;123(7):739–49. https://doi.org/10.1161/CIRCULATIONAHA.110.983353.
Sembill JA, Gerner ST, Volbers B, et al. Severity assessment in maximally treated ICH patients: The max-ICH score. Neurology. 2017;89(5):423–31. https://doi.org/10.1212/WNL.0000000000004174.
Strbian D, Meretoja A, Ahlhelm FJ, et al. Predicting outcome of IV thrombolysis-treated ischemic stroke patients: the DRAGON score. Neurology. 2012;78(6):427–32. https://doi.org/10.1212/WNL.0b013e318245d2a9.
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14–20. https://doi.org/10.3171/jns.1968.28.1.0014.
Alotaibi NM, Elkarim GA, Samuel N, et al. Effects of decompressive craniectomy on functional outcomes and death in poor-grade aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg. 2017;127(6):1315–25. https://doi.org/10.3171/2016.9.JNS161383.
Bailes JE, Spetzler RF, Hadley MN, Baldwin HZ. Management morbidity and mortality of poor-grade aneurysm patients. J Neurosurg. 1990;72(4):559–66. https://doi.org/10.3171/jns.1990.72.4.0559.
Brandecker S, Hadjiathanasiou A, Kern T, Schuss P, Vatter H, Guresir E. Primary decompressive craniectomy in poor-grade aneurysmal subarachnoid hemorrhage: long-term outcome in a single-center study and systematic review of literature. Neurosurg Rev. 2021;44(4):2153–62. https://doi.org/10.1007/s10143-020-01383-3.
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58. https://doi.org/10.1038/nrneurol.2013.246.
Zhao B, Rabinstein A, Murad MH, Lanzino G, Panni P, Brinjikji W. Surgical and endovascular treatment of poor-grade aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg Sci. 2017;61(4):403–15. https://doi.org/10.23736/S0390-5616.16.03457-3.
Witsch J, Frey HP, Patel S, et al. Prognostication of long-term outcomes after subarachnoid hemorrhage: The FRESH score. Ann Neurol. 2016;80(1):46–58. https://doi.org/10.1002/ana.24675.
Lee DH, Cho YS, Lee BK, et al. Late awakening is common in settings without withdrawal of life-sustaining therapy in out-of-hospital cardiac arrest survivors who undergo targeted temperature management. Crit Care Med. 2022;50(2):235–44. https://doi.org/10.1097/CCM.0000000000005274.
Rohaut B, Claassen J. Decision making in perceived devastating brain injury: a call to explore the impact of cognitive biases. Br J Anaesth. 2018;120(1):5–9. https://doi.org/10.1016/j.bja.2017.11.007.
Elmer J, Torres C, Aufderheide TP, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127–35. https://doi.org/10.1016/j.resuscitation.2016.01.016.
Turgeon AF, Lauzier F, Simard JF, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183(14):1581–8. https://doi.org/10.1503/cmaj.101786.
Steinberg A, Abella BS, Gilmore EJ, et al. Frequency of withdrawal of life-sustaining therapy for perceived poor neurologic prognosis. Crit Care Explor. 2021;3(7): e0487. https://doi.org/10.1097/CCE.0000000000000487.
Alkhachroum A, Bustillo AJ, Asdaghi N, et al. Withdrawal of life-sustaining treatment mediates mortality in patients with intracerebral hemorrhage with impaired consciousness. Stroke. 2021;52(12):3891–8. https://doi.org/10.1161/STROKEAHA.121.035233.
Asai A, Maekawa M, Akiguchi I, et al. Survey of Japanese physicians’ attitudes towards the care of adult patients in persistent vegetative state. J Med Ethics. 1999;25(4):302–8. https://doi.org/10.1136/jme.25.4.302.
Yaguchi A, Truog RD, Curtis JR, et al. International differences in end-of-life attitudes in the intensive care unit: results of a survey. Arch Intern Med. 2005;165(17):1970–5. https://doi.org/10.1001/archinte.165.17.1970.
Makino J, Fujitani S, Twohig B, Krasnica S, Oropello J. End-of-life considerations in the ICU in Japan: ethical and legal perspectives. J Intensive Care. 2014;2(1):9. https://doi.org/10.1186/2052-0492-2-9.
The Japanese Society of Intensive Care Medicine EC. The current situation survey about the clinical ethics in the affiliation facilities of the Japanese society of intensive care medicine councilor. J Jpn Soc Intensive Care Med. 2013;20:307–19.
(JSEPTIC) JSoPaTiIC. A brief survey for end-of-life. 2012 http://www.jseptic.com/rinsho/pdf/questionnaire_120125.pdf.
Akabayashi A, Slingsby BT, Kai I. Perspectives on advance directives in Japanese society: a population-based questionnaire survey. BMC Med Ethics. 2003;4:E5. https://doi.org/10.1186/1472-6939-4-5.
Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–85. https://doi.org/10.1089/neu.1998.15.573.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–7. https://doi.org/10.1161/01.str.19.5.604.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29.
Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure: on behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22(7):707–10. https://doi.org/10.1007/BF01709751.
Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46(6):660–2. https://doi.org/10.1001/archneur.1989.00520420080026.
Wilson L, Boase K, Nelson LD, et al. A Manual for the Glasgow outcome scale-extended interview. J Neurotrauma. 2021;38(17):2435–46. https://doi.org/10.1089/neu.2020.7527.
Fatima N, Razzaq S, El Beltagi A, Shuaib A, Saqqur M. Decompressive craniectomy: a preliminary study of comparative radiographic characteristics predicting outcome in malignant ischemic stroke. World Neurosurg. 2020;133:e267–74. https://doi.org/10.1016/j.wneu.2019.08.223.
Stienen MN, Visser-Meily JM, Schweizer TA, et al. Prioritization and timing of outcomes and endpoints after aneurysmal subarachnoid hemorrhage in clinical trials and observational studies: proposal of a multidisciplinary research group. Neurocrit Care. 2019;30(Suppl 1):102–13. https://doi.org/10.1007/s12028-019-00737-0.
Chen KY, Kung WM, Kuo LT, Huang AP. Ultrarapid endoscopic-aided hematoma evacuation in patients with thalamic hemorrhage. Behav Neurol. 2021;2021:8886004. https://doi.org/10.1155/2021/8886004.
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I: conceptual framework and item selection. Med Care. 1992;30(6):473–83.
Estraneo A, Moretta P, Loreto V, Lanzillo B, Santoro L, Trojano L. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology. 2010;75(3):239–45. https://doi.org/10.1212/WNL.0b013e3181e8e8cc.
Hammond FM, Giacino JT, Nakase Richardson R, et al. Disorders of consciousness due to traumatic brain injury: functional status ten years post-injury. J Neurotrauma. 2019;36(7):1136–46. https://doi.org/10.1089/neu.2018.5954.
Katz DI, Polyak M, Coughlan D, Nichols M, Roche A. Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1–4 year follow-up. Prog Brain Res. 2009;177:73–88. https://doi.org/10.1016/S0079-6123(09)17707-5.
Nakase-Richardson R, Whyte J, Giacino JT, et al. Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J Neurotrauma. 2012;29(1):59–65. https://doi.org/10.1089/neu.2011.1829.
Whyte J, Nakase-Richardson R, Hammond FM, et al. Functional outcomes in traumatic disorders of consciousness: 5-year outcomes from the National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems. Arch Phys Med Rehabil. 2013;94(10):1855–60. https://doi.org/10.1016/j.apmr.2012.10.041.
Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56(6):766–72. https://doi.org/10.1212/wnl.56.6.766.
Velly L, Perlbarg V, Boulier T, et al. Use of brain diffusion tensor imaging for the prediction of long-term neurological outcomes in patients after cardiac arrest: a multicentre, international, prospective, observational, cohort study. Lancet Neurol. 2018;17(4):317–26. https://doi.org/10.1016/S1474-4422(18)30027-9.
Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. 2019;380(26):2497–505. https://doi.org/10.1056/NEJMoa1812757.
Edlow BL, Chatelle C, Spencer CA, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain. 2017;140(9):2399–414. https://doi.org/10.1093/brain/awx176.
Kondziella D, Menon DK, Helbok R, et al. A precision medicine framework for classifying patients with disorders of consciousness: advanced classification of consciousness endotypes (ACCESS). Neurocrit Care. 2021;35(Suppl 1):27–36. https://doi.org/10.1007/s12028-021-01246-9.
Muehlschlegel S, Goostrey K, Flahive J, Zhang Q, Pach JJ, Hwang DY. A pilot randomized clinical trial of a goals-of-care decision aid for surrogates of severe acute brain injury patients. Neurology. 2022. https://doi.org/10.1212/WNL.0000000000200937.
Muehlschlegel S, Hwang DY, Flahive J, et al. Goals-of-care decision aid for critically ill patients with TBI: development and feasibility testing. Neurology. 2020;95(2):e179–93. https://doi.org/10.1212/WNL.0000000000009770.
Evans LR, Boyd EA, Malvar G, et al. Surrogate decision-makers’ perspectives on discussing prognosis in the face of uncertainty. Am J Respir Crit Care Med. 2009;179(1):48–53. https://doi.org/10.1164/rccm.200806-969OC.
White DB, Ernecoff N, Buddadhumaruk P, et al. Prevalence of and factors related to discordance about prognosis between physicians and surrogate decision makers of critically Ill patients. JAMA. 2016;315(19):2086–94. https://doi.org/10.1001/jama.2016.5351.
Zier LS, Burack JH, Micco G, et al. Doubt and belief in physicians’ ability to prognosticate during critical illness: the perspective of surrogate decision makers. Crit Care Med. 2008;36(8):2341–7. https://doi.org/10.1097/CCM.0b013e318180ddf9.
Wagner AK. TBI rehabilomics research: an exemplar of a biomarker-based approach to precision care for populations with disability. Curr Neurol Neurosci Rep. 2017;17(11):84. https://doi.org/10.1007/s11910-017-0791-5.
Muehlschlegel S, Shutter L, Col N, Goldberg R. Decision aids and shared decision-making in neurocritical care: an unmet need in our NeuroICUs. Neurocrit Care. 2015;23(1):127–30. https://doi.org/10.1007/s12028-014-0097-2.
Acknowledgements
We express our sincere thanks to Nestor Serrano for translating the medical record notes from Japanese into English (Supplemental Fig. 3). I would confirm that this manuscript has not been published elsewhere and is not under consideration by another journal.
Funding
JC is supported by grant funding from the National Institutes of Health (NS106014, R03 NS112760). SE is supported by a grant from Toda Medical Group Asaka Medical Center.
Author information
Authors and Affiliations
Contributions
SE, JA, and JC wrote the article. SE, SN, YF, SF, KM, AS, MO, YY, HA, SM, and HN were involved in data collection. QS performed data analysis. All authors contributed a critical and final revision of the article. The final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
There are no competing interests to report.
Ethical approval/informed consent
For this retrospective and prospective analysis, we sought approval from the ethics committee of the Toda Medical Group Asaka Medical Center (institutional review board [IRB]) to conduct this study (IRB 21-07). The IRB approved a waiver of participant consent for the retrospective review. For the prospective assessment of patient outcomes, we did obtain consent from the patient whenever possible and from the surrogate decision-maker if patients were unable to provide consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Egawa, S., Ader, J., Shen, Q. et al. Long-Term Outcomes of Patients with Stroke Predicted by Clinicians to have no Chance of Meaningful Recovery: A Japanese Cohort Study. Neurocrit Care 38, 733–740 (2023). https://doi.org/10.1007/s12028-022-01644-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01644-7