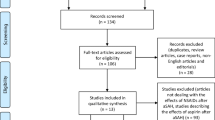
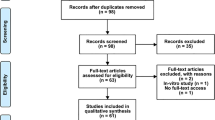
Abstract
Background
Cilostazol, a phosphodiesterase III inhibitor, appears to be a promising agent for preventing cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. Here, the authors perform a systematic review and meta-analysis to quantitatively assess the effects of cilostazol on brain structural and functional outcomes in animal models of cerebral ischemia and subarachnoid hemorrhage–induced cerebral vasospasm.
Methods
By using the PRISMA guidelines, a search of the PubMed, Scopus, and Web of Science was conducted to identify relevant studies. Study quality of each included study for both systematic reviews were scored by using an adapted 15-item checklist from the Collaborative Approach to Meta-Analysis of Animal Data from Experimental Studies. We calculated a standardized mean difference as effect size for each comparison. For each outcome, comparisons were combined by using random-effects modeling to account for heterogeneity, with a restricted maximum likelihood estimate of between-study variance.
Results
A total of 22 (median [Q1, Q3] quality score of 7 [5, 8]) and 6 (median [Q1, Q3] quality score of 6 [6, 6]) studies were identified for cerebral ischemia and subarachnoid hemorrhage–induced cerebral vasospasm, respectively. Cilostazol significantly reduced the infarct volume in cerebral ischemia models with a pooled standardized mean difference estimate of − 0.88 (95% confidence interval [CI] [− 1.07 to − 0.70], p < 0.0001). Cilostazol significantly reduced neurofunctional deficits in cerebral ischemia models with a pooled standardized mean difference estimate of − 0.66 (95% CI [− 1.06 to − 0.28], p < 0.0001). Cilostazol significantly improved the basilar artery diameter in subarachnoid hemorrhage–induced cerebral vasospasm with a pooled standardized mean difference estimate of 2.30 (95% CI [0.94 to 3.67], p = 0.001). Cilostazol also significantly improved the basilar artery cross-section area with a pooled standardized mean estimate of 1.88 (95% CI [0.33 to 3.43], p < 0.05). Overall, there was between-study heterogeneity and asymmetry in the funnel plot observed in all comparisons.
Conclusions
Published animal data support the overall efficacy of cilostazol in reducing infarct volume and neurofunctional deficits in cerebral ischemia models and cerebral vasospasm in subarachnoid hemorrhage models.







Similar content being viewed by others
References
Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. Stroke. 2012;43(6):1711–37. https://doi.org/10.1161/str.0b013e3182587839.
Qureshi AI, Lobanova I, Huang W, et al. Lessons learned from phase II and phase III trials investigating therapeutic agents for cerebral ischemia associated with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2022;36(2):662–81. https://doi.org/10.1007/s12028-021-01372-4.
Boulouis G, Labeyrie MA, Raymond J, et al. Treatment of cerebral vasospasm following aneurysmal subarachnoid haemorrhage: a systematic review and meta-analysis. Eur Radiol. 2017;27(8):3333–42. https://doi.org/10.1007/s00330-016-4702-y.
Vergouwen MD, Ilodigwe D, Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42(4):924–9. https://doi.org/10.1161/strokeaha.110.597914.
de Vries RBM, Hooijmans CR, Langendam MW, et al. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evidence-based Preclin Med. 2015;2(1): e00007. https://doi.org/10.1002/ebm2.7.
Vesterinen HM, Sena ES, Egan KJ, et al. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods. 2014;221:92–102. https://doi.org/10.1016/j.jneumeth.2013.09.010.
McCann SK, Cramond F, Macleod MR, Sena ES. Systematic review and meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke: an update. Transl Stroke Res. 2016;7(5):395–406. https://doi.org/10.1007/s12975-016-0489-z.
Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007;30(9):433–9. https://doi.org/10.1016/j.tins.2007.06.009.
Matsuda N, Naraoka M, Ohkuma H, et al. Effect of cilostazol on cerebral vasospasm and outcome in patients with aneurysmal subarachnoid hemorrhage: a randomized, double-blind. Placebo-Controlled Trial Cerebrovasc Dis. 2016;42(1–2):97–105. https://doi.org/10.1159/000445509.
Duval S, Tweedie R. A nonparametric, “Trim and Fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89–98. https://doi.org/10.1080/01621459.2000.10473905.
Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. https://doi.org/10.1136/bmj.d4002.
Bieber M, Schuhmann MK, Volz J, et al. Description of a novel phosphodiesterase (PDE)-3 inhibitor protecting mice from ischemic stroke independent from platelet function. Stroke. 2019;50(2):478–86. https://doi.org/10.1161/strokeaha.118.023664.
Choi JM, Shin HK, Kim KIY, Lee JH, Hong KIW. Neuroprotective effect of cilostazol against focal cerebral ischemia via antiapoptotic action in rats. J Pharmacol Exp Ther. 2002;300(3):787–93. https://doi.org/10.1124/jpet.300.3.787038.
Hase Y, Okamoto Y, Fujita Y, et al. Cilostazol, a phosphodiesterase inhibitor, prevents no-reflow and hemorrhage in mice with focal cerebral ischemia. Exp Neurol. 2012;233(1):523–33. https://doi.org/10.1016/j.expneurol.2011.11.038.
Honda F, Imai H, Ishikawa M, et al. Cilostazol attenuates gray and white matter damage in a rodent model of focal cerebral ischemia. Stroke. 2006;37(1):223–8. https://doi.org/10.1161/01.STR.0000196977.76702.6d.
Ishiguro M, Mishiro K, Fujiwara Y, et al. Phosphodiesterase-III inhibitor prevents hemorrhagic transformation induced by focal cerebral ischemia in mice treated with tPA. PLoS ONE. 2010;5(12):e15178. https://doi.org/10.1371/journal.pone.0015178.
Ito H, Hashimoto A, Matsumoto Y, Yao H, Miyakoda G. Cilostazol, a phosphodiesterase inhibitor, attenuates photothrombotic focal ischemic brain injury in hypertensive rats. J Cereb Blood Flow Metab. 2010;30(2):343–51. https://doi.org/10.1038/jcbfm.2009.220.
Kim JH, Hong KW, Bae SS, Shin YI, Choi BT, Shin HK. Probucol plus cilostazol attenuate hypercholesterolemia-induced exacerbation in ischemic brain injury via anti-inflammatory effects. Int J Mol Med. 2014;34(3):687–94. https://doi.org/10.3892/ijmm.2014.1848.
Kim JH, Park SH, Bae SS, et al. Combinatorial effect of probucol and cilostazol in focal ischemic mice with hypercholesterolemia. J Pharmacol Exp Ther. 2011;338(2):451–7. https://doi.org/10.1124/jpet.111.181180.
Kitashoji A, Egashira Y, Mishiro K, et al. Cilostazol ameliorates warfarin-induced hemorrhagic transformation after cerebral ischemia in mice. Stroke. 2013;44(10):2862–8. https://doi.org/10.1161/strokeaha.113.001183.
Lee JH, Kim KY, Lee YK, et al. Cilostazol prevents focal cerebral ischemic injury by enhancing casein kinase 2 phosphorylation and suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation in rats. J Pharmacol Exp Ther. 2004;308(3):896–903. https://doi.org/10.1124/jpet.103.061853.
Lee JH, Lee YK, Ishikawa M, et al. Cilostazol reduces brain lesion induced by focal cerebral ischemia in rats–an MRI study. Brain Res. 2003;994(1):91–8. https://doi.org/10.1016/j.brainres.2003.09.021.
Lee JH, Park SY, Lee WS, Hong KW. Lack of antiapoptotic effects of antiplatelet drug, aspirin and clopidogrel, and antioxidant, MCI-186, against focal ischemic brain damage in rats. Neurol Res. 2005;27(5):483–92. https://doi.org/10.1179/016164105x17134.
Lee JH, Park SY, Shin HK, Kim CD, Lee WS, Hong KW. Poly(ADP-ribose) polymerase inhibition by cilostazol is implicated in the neuroprotective effect against focal cerebral ischemic infarct in rat. Brain Res. 2007;1152:182–90. https://doi.org/10.1016/j.brainres.2007.03.035.
Nonaka Y, Koumura A, Hyakkoku K, et al. Combination treatment with normobaric hyperoxia and cilostazol protects mice against focal cerebral ischemia-induced neuronal damage better than each treatment alone. J Pharmacol Exp Ther. 2009;330(1):13–22. https://doi.org/10.1124/jpet.109.151548.
Nonaka Y, Tsuruma K, Shimazawa M, Yoshimura S, Iwama T, Hara H. Cilostazol protects against hemorrhagic transformation in mice transient focal cerebral ischemia-induced brain damage. Neurosci Lett. 2009;452(2):156–61. https://doi.org/10.1016/j.neulet.2009.01.039.
Oyama N, Yagita Y, Kawamura M, et al. Cilostazol, not aspirin, reduces ischemic brain injury via endothelial protection in spontaneously hypertensive rats. Stroke. 2011;42(9):2571–7. https://doi.org/10.1161/strokeaha.110.609834.
Park SY, Lee JH, Kim CD, Rhim BY, Hong KW, Lee WS. Beneficial synergistic effects of concurrent treatment with cilostazol and probucol against focal cerebral ischemic injury in rats. Brain Res. 2007;1157(1):112–20. https://doi.org/10.1016/j.brainres.2007.04.051.
Tanaka Y, Tanaka R, Liu M, Hattori N, Urabe T. Cilostazol attenuates ischemic brain injury and enhances neurogenesis in the subventricular zone of adult mice after transient focal cerebral ischemia. Neuroscience. 2010;171(4):1367–76. https://doi.org/10.1016/j.neuroscience.2010.10.008.
Toda Y, Katsura KI, Saito M, Inaba T, Sakurazawa M, Katayama Y. The effect of cilostazol and aspirin pre-treatment against subsequent transient focal cerebral ischemia in rat. Neurol Res. 2014;36(11):1011–9. https://doi.org/10.1179/1743132814Y.0000000389.
Wakida K, Morimoto N, Shimazawa M, et al. Cilostazol reduces ischemic brain damage partly by inducing metallothionein-1 and -2. Brain Res. 2006;1116(1):187–93. https://doi.org/10.1016/j.brainres.2006.07.125.
Ye YL, Shi WZ, Zhang WP, et al. Cilostazol, a phosphodiesterase 3 inhibitor, protects mice against acute and late ischemic brain injuries. Eur J Pharmacol. 2007;557(1):23–31. https://doi.org/10.1016/j.ejphar.2006.11.003.
Zhang Q, Ye YL, Li Q, et al. Protective effect of intranasal cilostazol administration on chronic injury after cerebral ischemia in mice. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2011;40(2):169–75.
Bilginer B, Onal B, Yiğitkanlı K, et al. Treatment of cerebral vasospasm with cilostazol in subarachnoid haemorrhage model. In: Cerebral vasospasm. Vienna: Springer. 2008. p. 291-295
Bilginer B, Onal MB, Narin F, Soylemezoglu F, Ziyal IM, Ozgen T. The effects of intravenous cilostazol and nimodipine on cerebral vasospasm after subarachnoid hemorrhage in an experimental rabbit model. Turk Neurosurg. 2009;19(4):374–9.
Ito H, Fukunaga M, Suzuki H, et al. Effect of cilostazol on delayed cerebral vasospasm after subarachnoid hemorrhage in rats: evaluation using black blood magnetic resonance imaging. Neurobiol Dis. 2008;32(1):157–61. https://doi.org/10.1016/j.nbd.2008.07.004.
Nishino A, Umegaki M, Fujinaka T, Yoshimine T. Cilostazol attenuates cerebral vasospasm after experimental subarachnoid hemorrhage. Neurol Res. 2010;32(8):873–8. https://doi.org/10.1179/016164109x12608733393791.
Onal MB, Bilginer B, Narin F, Ziyal MI, Soylemezoglu F, Ozgen T. Comparison of intrathecal cilostazol and nimodipine treatments in subarachnoid hemorrhage: an experimental study in rabbits. Acta Neurochir Suppl. 2011;110(2):43–8. https://doi.org/10.1007/978-3-7091-0356-2_9.
Yamaguchi-Okada M, Nishizawa S, Mizutani A, Namba H. Multifaceted effects of selective inhibitor of phosphodiesterase III, cilostazol, for cerebral vasospasm after subarachnoid hemorrhage in a dog model. Cerebrovasc Dis. 2009;28(2):135–42. https://doi.org/10.1159/000223439.
Zoerle T, Ilodigwe DC, Wan H, et al. Pharmacologic reduction of angiographic vasospasm in experimental subarachnoid hemorrhage: systematic review and meta-analysis. J Cereb Blood Flow Metab. 2012;32(9):1645–58. https://doi.org/10.1038/jcbfm.2012.57.
Dreier JP, Major S, Manning A, et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132(Pt 7):1866–81. https://doi.org/10.1093/brain/awp102.
Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008;28(11):1761–70. https://doi.org/10.1038/jcbfm.2008.74.
Reid JL, Dawson D, Macrae IM. Endothelin, cerebral ischaemia and infarction. Clin Exp Hypertens. 1995;17(1–2):399–407. https://doi.org/10.3109/10641969509087080.
Robinson MJ, Macrae IM, Todd M, Reid JL, McCulloch J. Reduction of local cerebral blood flow to pathological levels by endothelin-1 applied to the middle cerebral artery in the rat. Neurosci Lett. 1990;118(2):269–72. https://doi.org/10.1016/0304-3940(90)90644-o.
Liu GJ, Luo J, Zhang LP, et al. Meta-analysis of the effectiveness and safety of prophylactic use of nimodipine in patients with an aneurysmal subarachnoid haemorrhage. CNS Neurol Disord Drug Targets. 2011;10(7):834–44. https://doi.org/10.2174/187152711798072383.
Horn J, Haan RJD, Vermeulen M, Luiten PGM, Limburg M. Nimodipine in animal model experiments of focal cerebral ischemia. Stroke. 2001;32(10):2433–8. https://doi.org/10.1161/hs1001.096009.
van der Worp HB, Howells DW, Sena ES, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7(3):e1000245. https://doi.org/10.1371/journal.pmed.1000245.
Hu X, Zhu Y, Zhou F, Peng C, Hu Z, Chen C. Efficacy of melatonin in animal models of subarachnoid hemorrhage: a systematic review and stratified meta-analysis. Front Neurol. 2021;12:685731. https://doi.org/10.3389/fneur.2021.685731.
Tan J, Song R, Luo S, et al. Efficacy of resveratrol in experimental subarachnoid hemorrhage animal models: a stratified meta-analysis. Front Pharmacol. 2022;13:905208. https://doi.org/10.3389/fphar.2022.905208.
He J, Liu J, Huang Y, Lan Z, Tang X, Hu Z. Mesenchymal stem cells-derived therapies for subarachnoid hemorrhage in preclinical rodent models: a meta-analysis. Stem Cell Res Ther. 2022;13(1):42. https://doi.org/10.1186/s13287-022-02725-2.
Aliena-Valero A, Baixauli-Martín J, Castelló-Ruiz M, Torregrosa G, Hervás D, Salom JB. Effect of uric acid in animal models of ischemic stroke: A systematic review and meta-analysis. J Cereb Blood Flow Metab. 2021;41(4):707–22. https://doi.org/10.1177/0271678x20967459.
Gibson CL, Gray LJ, Murphy SP, Bath PMW. Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab. 2006;26(9):1103–13. https://doi.org/10.1038/sj.jcbfm.9600270.
Minnerup J, Heidrich J, Wellmann J, Rogalewski A, Schneider A, Schäbitz W-R. Meta-analysis of the efficacy of granulocyte-colony stimulating factor in animal models of focal cerebral ischemia. Stroke. 2008;39(6):1855–61. https://doi.org/10.1161/STROKEAHA.107.506816.
Davis CK, Laud PJ, Bahor Z, Rajanikant GK, Majid A. Systematic review and stratified meta-analysis of the efficacy of carnosine in animal models of ischemic stroke. J Cereb Blood Flow Metab. 2016;36(10):1686–94. https://doi.org/10.1177/0271678x16658302.
Chen L, Zhang G, Gu Y, Guo X. Meta-analysis and systematic review of neural stem cells therapy for experimental ischemia stroke in preclinical studies. Sci Rep. 2016;6(1):32291. https://doi.org/10.1038/srep32291.
Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke. 2008;39(10):2824–9. https://doi.org/10.1161/strokeaha.108.515957.
Vu Q, Xie K, Eckert M, Zhao W, Cramer SC. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82(14):1277–86. https://doi.org/10.1212/wnl.0000000000000278.
Crossley NA, Sena E, Goehler J, et al. Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke. 2008;39(3):929–34. https://doi.org/10.1161/strokeaha.107.498725.
Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–29. https://doi.org/10.1016/s0895-4356(00)00242-0.
Laban KG, Vergouwen MD, Dijkhuizen RM, et al. Effect of endothelin receptor antagonists on clinically relevant outcomes after experimental subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2015;35(7):1085–9. https://doi.org/10.1038/jcbfm.2015.89.
Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1). Stroke. 2008;39(11):3015–21. https://doi.org/10.1161/STROKEAHA.108.519942.
Qureshi AI, Ishfaq A, Ishfaq MF, et al. Therapeutic benefit of cilostazol in patients with aneurysmal subarachnoid hemorrhage: a meta-analysis of randomized and nonrandomized studies. J Vasc Interv Neurol. 2018;10(2):33–40.
Liu J, He J, Chen X, et al. Cilostazol for aneurysmal subarachnoid hemorrhage: an updated systematic review and meta-analysis. Cerebrovasc Dis. 2022;51(2):138–48. https://doi.org/10.1159/000518731.
Suzuki H, Nakatsuka Y, Yasuda R, et al. Dose-dependent inhibitory effects of cilostazol on delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. Transl Stroke Res. 2019;10(4):381–8. https://doi.org/10.1007/s12975-018-0650-y.
Kamada N, Yamada K, Odomi M, et al. Sex differences in pharmacokinetics of cilostazol in rats. Xenobiotica. 2011;41(10):903–13. https://doi.org/10.3109/00498254.2011.590242.
Akiyama H, Kudo S, Shimizu T. The absorption, distribution and excretion of a new antithrombotic and vasodilating agent, cilostazol, in rat, rabbit, dog and man. Arzneimittelforschung. 1985;35(7a):1124–32.
Schrör K. The pharmacology of cilostazol. Diabetes Obes Metab 2002;4 Suppl 2:S14–9. https://doi.org/10.1046/j.1463-1326.2002.0040s2s14.x.
Akiyama H, Kudo S, Shimizu T. The metabolism of a new antithrombotic and vasodilating agent, cilostazol, in rat, dog and man. Arzneimittelforschung. 1985;35(7a):1133–40.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
AIQ: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, reviewed submitted version of manuscript, administrative/technical/material support, and study supervision. INA: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, and reviewed submitted version of manuscript. XM: statistical analysis, conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, and reviewed submitted version of manuscript. AL: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, and reviewed submitted version of manuscript. IB: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, and reviewed submitted version of manuscript. JB: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, and reviewed submitted version of manuscript. JPB: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, and reviewed submitted version of manuscript. CNC: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, and reviewed submitted version of manuscript. RHM: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, and reviewed submitted version of manuscript. RS: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, and reviewed submitted version of manuscript. MT: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, and reviewed submitted version of manuscript. JIS: conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critically revising the article, reviewed submitted version of manuscript, administrative/technical/material support, and study supervision. All authors approved the final manuscript prior to its submission.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical Approval/Informed Consent
The manuscript adheres to ethical guidelines and consists of systematic review and meta-analysis of previously published data and therefore was not subject to ethical approvals (institutional review board) and/or use of informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qureshi, A.I., Akhtar, I.N., Ma, X. et al. Effect of Cilostazol in Animal Models of Cerebral Ischemia and Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis. Neurocrit Care 38, 698–713 (2023). https://doi.org/10.1007/s12028-022-01637-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01637-6