Abstract
Background
Science continues to search for a neuroprotective drug therapy to improve outcomes after cardiac arrest (CA). The use of glibenclamide (GBC) has shown promise in preclinical studies, but its effects on neuroprognostication tools are not well understood. We aimed to investigate the effect of GBC on somatosensory evoked potential (SSEP) waveform recovery post CA and how this relates to the early prediction of functional outcome, with close attention to arousal and somatosensory recovery, in a rodent model of CA.
Methods
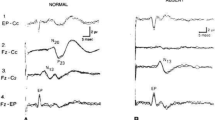
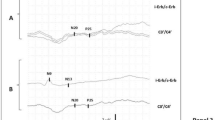
Sixteen male Wistar rats were subjected to 8-min asphyxia CA and assigned to GBC treatment (n = 8) or control (n = 8) groups. GBC was administered as a loading dose of 10 μg/kg intraperitoneally 10 min after the return of spontaneous circulation, followed by a maintenance dosage of 1.6 μg/kg every 8 h for 24 h. SSEPs were recorded from baseline until 150 min following CA. Coma recovery, arousal, and brainstem function, measured by subsets of the neurological deficit score (NDS), were compared between both groups. SSEP N10 amplitudes were compared between the two groups at 30, 60, 90, and 120 min post CA.
Results
Rats treated with GBC had higher sub-NDS scores post CA, with improved arousal and brainstem function recovery (P = 0.007). Both groups showed a gradual improvement of SSEP N10 amplitude over time, from 30 to 120 min post CA. Rats treated with GBC showed significantly better SSEP recovery at every time point (P < 0.001 for 30, 60, and 90 min; P = 0.003 for 120 min). In the GBC group, the N10 amplitude recovered to baseline by 120 min post CA. Quantified Cresyl violet staining revealed a significantly greater percentage of damage in the control group compared with the GBC treatment group (P = 0.004).
Conclusions
Glibenclamide improves coma recovery, arousal, and brainstem function after CA with decreased number of ischemic neurons in a rat model. GBC improves SSEP recovery post CA, with N10 amplitude reaching the baseline value by 120 min, suggesting early electrophysiologic recovery with this treatment. This medication warrants further exploration as a potential drug therapy to improve functional outcomes in patients after CA.





Similar content being viewed by others
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-528.
Green CR, Botha JA, Tiruvoipati R. Cognitive function, quality of life and mental health in survivors of our-of-hospital cardiac arrest: a review. Anaesth Intensive Care. 2015;43(5):568–76.
Perman SM, Goyal M, Neumar RW, Topjian AA, et al. Clinical applications of targeted temperature management. Chest. 2014;145(2):386–93.
Bernard SA, Gray TW, Buist MD, Jones BM, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63.
Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56.
Taccone FS, Picetti E, Vincent JL. High quality targeted temperature management (TTM) after cardiac arrest. Crit Care. 2020;24(1):6.
Caffes N, Kurland DB, Gerzanich V, Simard JM. Glibenclamide for the treatment of ischemic and hemorrhagic stroke. Int J Mol Sci. 2015;16(3):4973–84.
Mehta RI, Tosun C, Ivanova S, Tsymbalyuk N, et al. Sur1-Trpm4 cation channel expression in human cerebral infarcts. J Neuropathol Exp Neurol. 2015;74(8):835–49.
Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: a focused review. J Cereb Blood Flow Metab. 2012;32(9):1699–717.
Simard JM, Sheth KN, Kimberly WT, Stern BJ, et al. Glibenclamide in cerebral ischemia and stroke. Neurocrit Care. 2014;20(2):319–33.
Zweckberger K, Hackenberg K, Jung CS, Hertle DN, et al. Glibenclamide reduces secondary brain damage after experimental traumatic brain injury. Neuroscience. 2014;272:199–206.
Huang K, Wang Z, Gu Y, Ji Z, et al. Glibenclamide prevents water diffusion abnormality in the brain after cardiac arrest in rats. Neurocrit Care. 2018;29(1):128–35.
Huang K, Gu Y, Hu Y, Ji Z, et al. Glibenclamide improves survival and neurologic outcome after cardiac arrest in rats. Crit Care Med. 2015;43(9):e341–9.
Madhok J, Maybhate A, Xiong W, Koenig MA, et al. Quantitative assessment of somatosensory-evoked potentials after cardiac arrest in rats: prognostication of functional outcomes. Crit Care Med. 2010;38(8):1709–17.
Xiong W, Koenig MA, Madhok J, Jia X, et al. Evolution of Somatosensory evoked potentials after cardiac arrest induced hypoxic-ischemic injury. Resuscitation. 2010;81(7):893–7.
Jia X, Koenig MA, Nickl R, Zhen G, et al. Early electrophysiologic markers predict functional outcome associated with temperature manipulation after cardiac arrest in rats. Crit Care Med. 2008;36(6):1909–16.
Jia X, Koenig MA, Shin HC, Zhen G, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008;76(3):431–42.
Jia X, Koenig MA, Shin HC, Zhen G, et al. Quantitative EEG and neurological recovery with therapeutic hypothermia after asphyxial cardiac arrest in rats. Brain Res. 2006;1111(1):166–75.
Wang Z, Du J, Lachance BB, Mascarenhas C, et al. Intracerebroventricular administration of hNSCs improves neurological recovery after cardiac arrest in rats. Stem Cell Rev Rep. 2020. Accessed
He J, Lu H, Young L, Deng R, et al. Real-time quantitative monitoring of cerebral blood flow by laser speckle contrast imaging after cardiac arrest with targeted temperature management. J Cereb Blood Flow Metab. 2019;39(6):1161–71.
Deng R, Koenig MA, Young LM, Jia X. Early quantitative gamma-band EEG marker is associated with outcomes after cardiac arrest and targeted temperature management. Neurocrit Care. 2015;23(2):262–73.
Deng R, Young LM, Jia X. Quantitative EEG markers in severe post-resuscitation brain injury with therapeutic hypothermia. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:6598–601.
Simard JM, Woo SK, Tsymbalyuk N, Voloshyn O, et al. Glibenclamide-10-h treatment window in a clinically relevant model of stroke. Transl Stroke Res. 2012;3(2):286–95.
Shin HC, Tong S, Yamashita S, Jia X, et al. Quantitative EEG and effect of hypothermia on brain recovery after cardiac arrest. IEEE Trans Biomed Eng. 2006;53(6):1016–23.
Madhok J, Wu D, Xiong W, Geocadin RG, et al. Hypothermia amplifies somatosensory-evoked potentials in uninjured rats. J Neurosurg Anesthesiol. 2012;24(3):197–202.
Dragancea I, Horn J, Kuiper M, Friberg H, et al. Neurological prognostication after cardiac arrest and targeted temperature management 33 degrees C versus 36 degrees C: results from a randomised controlled clinical trial. Resuscitation. 2015;93:164–70.
Sandroni C, Cavallaro F, Callaway CW, D’Arrigo S, et al. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 2: patients treated with therapeutic hypothermia. Resuscitation. 2013;84(10):1324–38.
Bouwes A, Binnekade JM, Kuiper MA, Bosch FH, et al. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Ann Neurol. 2012;71(2):206–12.
Bouwes A, Binnekade JM, Verbaan BW, Zandbergen EG, et al. Predictive value of neurological examination for early cortical responses to somatosensory evoked potentials in patients with postanoxic coma. J Neurol. 2012;259(3):537–41.
Rossetti AO, Rabinstein AA, Oddo M. Neurological prognostication of outcome in patients in coma after cardiac arrest. Lancet Neurol. 2016;15(6):597–609.
Maciel CB, Morawo AO, Tsao CY, Youn TS, et al. SSEP in therapeutic hypothermia era. J Clin Neurophysiol. 2017;34(5):469–75.
Lachance B, Wang Z, Badjatia N, Jia X. Somatosensory evoked potentials and neuroprognostication after cardiac arrest. Neurocrit Care. 2020;32(3):847–57.
Young LM CRJX. Multimodel quantitative analysis of somatosensory evoked potentials after cardiac arrest with graded hypothermia. Conf Proc IEEE Eng Med Biol Soc. 2017:1846–9.
Glimmerveen AKH, Ruijter B, Tjepkema-Cloostermans M, van Putten M, Hofmeijer J. Relevance of Somatosensory evoked potential amplitude after cardiac arrest. Front Neurol. 2020;11:335.
Oh SH, Park KN, Choi SP, Oh JS, et al. Beyond dichotomy: patterns and amplitudes of SSEPs and neurological outcomes after cardiac arrest. Crit Care. 2019;23(1):224.
Kurland DB. Glibenclamide for the treatment of acute CNS injury. Pharmaceuticals. 2013;6(10):1287–303.
Huang K, Wang Z, Gu Y, Hu Y, et al. Glibenclamide is comparable to target temperature management in improving survival and neurological outcome after asphyxial cardiac arrest in rats. J Am Heart Assoc. 2016;5(7):e003465.
Simard JM. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic injury. Nat Med. 2006;12(4):433–40.
Nakano T, Hurn PD, Herson PS, Traystman RJ. Testosterone exacerbates neuronal damage following cardiac arrest and cardiopulmonary resuscitation in mouse. Brain Res. 2010;1357:124–30.
Oh SH, Park KN, Lim J, Choi SP, et al. The impact of sex and age on neurological outcomes in out-of-hospital cardiac arrest patients with targeted temperature management. Crit Care. 2017;21(1):272.
Pergakis M, Badjatia N, Chaturvedi S, Cronin CA, et al. BIIB093 (IV glibenclamide): an investigational compound for the prevention and treatment of severe cerebral edema. Expert Opin Investig Drugs. 2019;28(12):1031–40.
Funding
The work was supported by R01HL118084 and R01NS110387 from the United States National Institutes of Health (both to XJ). XJ was partially supported by the National Institutes of Health RO1 NS117102 (to XJ).
Author information
Authors and Affiliations
Contributions
Brittany Bolduc Lachance analyzed the data and wrote the article and worked on the revision, Zhuoran Wang performed the in vivo study and related in vitro study. Neeraj Badjatia provided critical appraisal. Xiaofeng Jia conceived the original idea, designed the experiments, revised, and finalized the article. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Human and animal rights
Experimental protocols were approved by the University of Maryland Baltimore Institutional Animal Care and Use Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lachance, B.B., Wang, Z., Badjatia, N. et al. The effect of Glibenclamide on somatosensory evoked potentials after cardiac arrest in rats. Neurocrit Care 36, 612–620 (2022). https://doi.org/10.1007/s12028-021-01350-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01350-w