Abstract
Background
Inducing normothermia with surface cooling temperature modulating devices (TMDs) is cumbersome and often associated with significant shivering. We tested the safety and feasibility of a novel transnasal evaporative cooling device to induce and maintain normothermia in febrile patients following ischemic and hemorrhagic stroke.
Methods
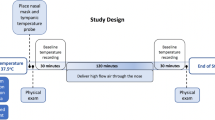
A single-center study utilizing the CoolStat® transnasal cooling device was used to achieve core temperature reduction in mechanically ventilated stroke patients with fever (T ≥ 38.3 C) refractory to acetaminophen by inducing an evaporative cooling energy exchange in the nasal turbinates thru a high flow of dehumidified air into the nasal cavity and out through the mouth. Continuous temperature measurements were obtained from tympanic and core (esophageal or bladder) temperature monitors. Safety assessments included continuous monitoring for hypertension, tachycardia, and raised intracranial pressure (when monitored). Otolaryngology (ENT) evaluations were monitored for any device-related nasal mucosal injury with a pre- and post-visual examination. Shivering was assessed every 30 min using the Bedside Shivering Assessment Scale (BSAS). Duration of device use was limited to 8 h, at which time patients were transitioned to routine care for temperature management.
Results
Ten subjects (median age: 54 years, BMI: 32.5 kg/m2, 60% men) were enrolled with normothermia achieved in 90% of subjects. One subject did not achieve normothermia and was later refractory to other TMDs. Median baseline temperature was 38.5 ± 0.1 C, with a reduction noted by 4 h (38.5 ± 0.1 vs 37.3 ± 0.8, P < 0.001) and sustained at 8 h (38.5 ± 0.1 vs 37.1 ± 0.7, P = 0.001). Time to normothermia was 2.6 ± 1.9 h. The median BSAS was 0 (range 0–1) with only 4 episodes necessitating meperidine across 76 h of study monitoring. No treatment was discontinued due to safety concerns. ENT evaluations noted no device-related adverse findings.
Conclusions
Inducing normothermia with a novel transnasal TMD appears to be safe, feasible and not associated with significant shivering. A multicenter trial testing the ability of the CoolStat to maintain normothermia for 24 h is currently underway.



Similar content being viewed by others
References
Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603.
Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke. 2008;39:3029–35.
Scaravilli V, Tinchero G, Citerio G. Participants in the international multi-disciplinary consensus conference on the Critical Care Management of Subarachnoid H. Fever management in SAH. Neurocrit Care. 2011;15:287–94.
Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418.
Gowda R, Jaffa M, Badjatia N. Thermoregulation in brain injury. Handb Clin Neurol. 2018;157:789–97.
Khan I, Haymore J, Barnaba B, et al. Esophageal cooling device versus other temperature modulation devices for therapeutic normothermia in subarachnoid and intracranial hemorrhage. Ther Hypothermia Temp Manag. 2018;8:53–8.
Mayer SA, Kowalski RG, Presciutti M, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med. 2004;32:2508–15.
Diringer MN. Neurocritical care fever reduction trial G. Treatment of fever in the neurologic intensive care unit with a catheter-based heat exchange system. Crit Care Med. 2004;32:559–64.
Schmutzhard E, Engelhardt K, Beer R, et al. Safety and efficacy of a novel intravascular cooling device to control body temperature in neurologic intensive care patients: a prospective pilot study. Crit Care Med. 2002;30:2481–8.
Griffiths SA, Ahmad J, Francoeur CL, et al. The EMCOOLs surface cooling system for fever control in neurocritical care patients: a pilot study. Clin Neurol Neurosurg. 2019;184:105412.
Badjatia N, Kowalski RG, Schmidt JM, et al. Predictors and clinical implications of shivering during therapeutic normothermia. Neurocrit Care. 2007;6:186–91.
Badjatia N, Strongilis E, Gordon E, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008;39:3242–7.
De Witte J, Sessler DI. Perioperative shivering: physiology and pharmacology. Anesthesiology. 2002;96:467–84.
Hata JS, Shelsky CR, Hindman BJ, Smith TC, Simmons JS, Todd MM. A prospective, observational clinical trial of fever reduction to reduce systemic oxygen consumption in the setting of acute brain injury. Neurocrit Care. 2008;9:37–44.
Lyden P, Hemmen T, Grotta J, et al. Results of the ICTuS 2 Trial (Intravascular Cooling in the Treatment of Stroke 2). Stroke. 2016;47:2888–955.
Oddo M, Frangos S, Maloney-Wilensky E, Andrew Kofke W, Le Roux PD, Levine JM. Effect of shivering on brain tissue oxygenation during induced normothermia in patients with severe brain injury. Neurocrit Care. 2010;12:10–6.
Presciutti M, Bader MK, Hepburn M. Shivering management during therapeutic temperature modulation: nurses' perspective. Crit Care Nurse. 2012;32:33–42.
Lord AS, Karinja S, Lantigua H, et al. Therapeutic temperature modulation for fever after intracerebral hemorrhage. Neurocrit Care. 2014;21:200–6.
Badjatia N, O'Donnell J, Baker JR, et al. Achieving normothermia in patients with febrile subarachnoid hemorrhage: feasibility and safety of a novel intravascular cooling catheter. Neurocrit Care. 2004;1:145–56.
Choi HA, Ko SB, Presciutti M, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care. 2011;14:389–94.
Assis FR, Bigelow MEG, Chava R, et al. Efficacy and safety of transnasal CoolStat cooling device to induce and maintain hypothermia. Ther Hypothermia Temp Manag. 2019;9:108–17.
Madden LK, Hill M, May TL, et al. The implementation of targeted temperature management: an evidence-based guideline from the Neurocritical Care Society. Neurocrit Care. 2017;27:468–87.
Jarrah S, Dziodzio J, Lord C, et al. Surface cooling after cardiac arrest: effectiveness, skin safety, and adverse events in routine clinical practice. Neurocrit Care. 2011;14:382–8.
Chava R, Zviman M, Raghavan MS, et al. Rapid induction of therapeutic hypothermia using transnasal high flow dry air. Therap Hypothermia Temp Manag. 2017;7:50–6.
Castren M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation. 2010;122:729–36.
Grave MS, Sterz F, Nurnberger A, et al. Safety and feasibility of the RhinoChill immediate transnasal evaporative cooling device during out-of-hospital cardiopulmonary resuscitation: a single-center, observational study. Medicine (Baltimore). 2016;95:e4692.
Harris S, Bansbach J, Dietrich I, Kalbhenn J, Schmutz A. RhinoChill((R))-more than an "ice-cream headache (1)" serious adverse event related to transnasal evaporative cooling. Resuscitation. 2016;103:e5–e6.
Ziai WC, Shah D, Assis FR, Tandri H, Geocadin RG. Feasibility and safety of transnasal high flow air to reduce core body temperature in febrile neurocritical care patients: a pilot study. Neurocrit Care. 2019;31:280–7.
Funding
This study was funded by the Maryland Industrial Partnerships (MIPS) program.
Author information
Authors and Affiliations
Contributions
Conception of the study design done by NB and HT. Data collection was done by JH, SS, RS, and HT. Data analysis and interpretation was done by NB. Drafting of the article was done by NB, SS, and JH. Critical revision of the manuscript was done by CH, HT, NB, JH. All authors approved the final version of this manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
Harikrishna Tandri is the founder of CoolTech LLC, which is developing a transnasal device for hypothermia. The other authors report no conflict of interests.
Ethical approval/informed consent
The study was approved by the University of Maryland, Baltimore Institutional Review Board. Informed consent for participation in the study was obtained from the legally authorized representative for each study patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Badjatia, N., Gupta, N., Sanchez, S. et al. Safety and Feasibility of a Novel Transnasal Cooling Device to Induce Normothermia in Febrile Cerebrovascular Patients. Neurocrit Care 34, 500–507 (2021). https://doi.org/10.1007/s12028-020-01044-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-020-01044-9