Abstract
Background
Critically ill aneurysmal subarachnoid hemorrhage (aSAH) patients suffer from systemic complications at a high rate. Hyperglycemia is a common intensive care unit (ICU) complication and has become a focus after aggressive glucose management was associated with improved ICU outcomes. Subsequent research has suggested that glucose variability, not a specific blood glucose range, may be a more appropriate clinical target. Glucose variability is highly correlated to poor outcomes in a wide spectrum of critically ill patients. Here, we investigate the changes between subsequent glucose values termed “inter-measurement difference,” as an indicator of glucose variability and its association with outcomes in patients with aSAH.
Methods
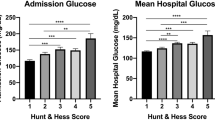
All SAH admissions to a single, tertiary referral center between 2002 and 2016 were screened. All aneurysmal cases who had more than 2 glucose measurements were included (n = 2451). We calculated several measures of variability, including simple variance, the average consecutive absolute change, average absolute change by time difference, within subject variance, median absolute deviation, and average or median consecutive absolute percentage change. Predictor variables also included admission Hunt and Hess grade, age, gender, cardiovascular risk factors, and surgical treatment. In-patient mortality was the main outcome measure.
Results
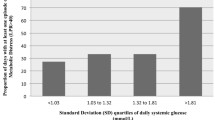
In a multiple regression analysis, nearly all forms of glucose variability calculations were found to be correlated with in-patient mortality. The consecutive absolute percentage change, however, was most predictive: OR 5.2 [1.4–19.8, CI 95%] for percentage change and 8.8 [1.8–43.6] for median change, when controlling for the defined predictors. Survival to ICU discharge was associated with lower glucose variability (consecutive absolute percentage change 17% ± 9%) compared with the group that did not survive to discharge (20% ± 15%, p < 0.01). Interestingly, this finding was not significant in patients with pre-admission poorly controlled diabetes as indicated by HbA1c (OR 0.45 [0.04–7.18], by percentage change). The effect is driven mostly by non-diabetic patients or those with well-controlled diabetes.
Conclusions
Reduced glucose variability is highly correlated with in-patient survival and long-term mortality in aSAH patients. This finding was observed in the non-diabetic and well-controlled diabetic patients, suggesting a possible benefit for personalized glucose targets based on baseline HbA1c and minimizing variability. The inter-measure percentage change as an indicator of glucose variability is not only predictive of outcome, but is an easy-to-use tool that could be implemented in future clinical trials.



Similar content being viewed by others
References
Chi A, Lissauer ME, Kirchoffner J, Scalea TM, Johnson SB. Effect of glycemic state on hospital mortality in critically ill surgical patients. Am Surg. 2011;77:1483–9.
Oddo M, Schmidt JM, Mayer SA, Chiolero RL. Glucose control after severe brain injury. Curr Opin Clin Nutr Metab Care. 2008;11:134–9.
Hermanides J, Plummer MP, Finnis M, Deane AM, Coles JP, Menon DK. Glycaemic control targets after traumatic brain injury: a systematic review and meta-analysis. Crit Care. 2018;22:1–11.
Frontera JA, Fernandez A, Claassen J, Schmidt M, Schumacher HC, Wartenberg K, et al. Hyperglycemia after SAH: predictors, associated complications, and impact on outcome. Stroke. 2006;37:199–203.
Rincon F, Rossenwasser RH, Dumont A. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery. 2013;73:217–22.
Finfer S, Chittock DR, Su S, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2008;360:1283–97.
Hersh AM, Hirshberg EL, Wilson EL, Orme JF, Morris AH, Lanspa MJ. Lower glucose target is associated with improved 30-day mortality in cardiac and cardiothoracic patients. Chest. 2018;154:1044–51.
Preiser JC, Lheureux O, Thooft A, Brimioulle S, Goldstein J, Vincent JL. Near-continuous glucose monitoring makes glycemic control safer in ICU patients. Crit Care Med. 2018;46:1224–9.
Kurtz P, Claassen J, Schmidt JM, Helbok R, Hanafy KA, Presciutti M, et al. Reduced brain/serum glucose ratios predict cerebral metabolic distress and mortality after severe brain injury. Neurocrit Care. 2013;19:311–9.
Zetterling M, Hillered L, Enblad P, Karlsson T, Ronne-Engström E. Relation between brain interstitial and systemic glucose concentrations after subarachnoid hemorrhage. J Neurosurg. 2011;115:66–74.
Helbok R, Schmidt JM, Kurtz P, Hanafy KA, Fernandez L, Stuart RM, et al. Systemic glucose and brain energy metabolism after subarachnoid hemorrhage. Neurocrit Care. 2010;12:317–23.
Godin PJ, Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 1996;24(7):1107–16.
Matsushima K, Peng M, Velasco C, Schaefer E, Diaz-Arrastia R, Frankel H. Glucose variability negatively impacts long-term functional outcome in patients with traumatic brain injury. J Crit Care. 2012;27:125–31.
Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008–13.
Barletta JF, Figueroa BE, DeShane R, Blau SA, McAllen KJ. High glucose variability increases cerebral infarction in patients with spontaneous subarachnoid hemorrhage. J Crit Care. 2013;28:798–803.
Stawicki SP, Schuster D, Liu JF, et al. The glucogram: a new quantitative tool for glycemic analysis in the surgical intensive care unit. Int J Crit Illn Inj Sci. 2011;1(1):5–12.
Ruppert D. Statistics and data analysis for financial engineering. New York: Springer; 2011.
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20.
Stroux L, Redman CW, Georgieva A, Payne SJ, Clifford GD. Doppler-based fetal heart rate analysis markers for the detection of early intrauterine growth restriction. Acta Obstet Gynecol Scand. 2017;96:1322–9.
Wertheimer PE, Jouvet M, Descotes J. Diagnosis of death of the nervous system in comas with respiratory arrest treated by artificial respiration. Presse Med. 1959;67:87–8.
Moss TJ, Calland JF, Enfield KB, Gomez-Manjarres DC, Ruminski C, DiMarco JP, et al. New-onset atrial fibrillation in the critically ill. Crit Care Med. 2017;45:790–7.
Wang C, Lv L, Yang Y, Chen D, Liu G, Chen L, et al. Glucose fluctuations in subjects with normal glucose tolerance, impaired glucose regulation and newly diagnosed type 2 diabetes mellitus. Clin Endocrinol. 2012;76:810–5.
Borgquist O, Wise MP, Nielsen N, Al-Subaie N, Cranshaw J, Cronberg T, et al. Dysglycemia, glycemic variability, and outcome after cardiac arrest and temperature management at 33°C and 36°C. Crit Care Med. 2017;45:1337–43.
Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiol J Am Soc Anesthesiol. 2006;105:244–52.
Chase JG, Le Compte AJ, Suhaimi F, Shaw GM, Lynn A, Lin J, et al. Tight glycemic control in critical care—the leading role of insulin sensitivity and patient variability: a review and model-based analysis. Comput Methods Programs Biomed. 2011;102:156–71.
Ali NA, O’Brien JM, Dungan K, Phillips G, Marsh CB, Lemeshow S, et al. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316–21.
Beseoglu K, Steiger HJ. Elevated glycated hemoglobin level and hyperglycemia after aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 2017;163:128–32.
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–32.
Luethi N, Cioccari L, Crisman M, Bellomo R, Eastwood GM, Mårtensson J. Prevalence of ketosis, ketonuria, and ketoacidosis during liberal glycemic control in critically ill patients with diabetes: an observational study. Crit Care. 2016;20:297.
Luethi N, Cioccari L, Biesenbach P, Lucchetta L, Kagaya H, Morgan R, et al. Liberal glucose control in ICU patients with diabetes: a before-and-after study. Crit Care Med. 2018;46:935–42.
Di Muzio F, Presello B, Glassford NJ, Tsuji IY, Eastwood GM, Deane AM, et al. Liberal versus conventional glucose targets in critically ill diabetic patients: an exploratory safety cohort assessment. Crit Care Med. 2016;44:1683–91.
Johnston KC, Bruno A, Pauls Q, Hall CE, Barrett KM, Barsan W, et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE randomized clinical trial. JAMA. 2019;322:326–35.
Lanspa MJ, Krinsley JS, Hersh AM, Wilson EL, Holmen JR, Orme JF, et al. Percentage of time in range 70 to 139 mg/dL is associated with reduced mortality among critically ill patients receiving IV insulin infusion. Chest. 2019;156:878–86.
Oddo M, Schmidt JM, Carrera E, Badjatia N, Connolly ES, Presciutti M, et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med. 2008;16(12):3233–8.
Funding
There was no support for this work.
Author information
Authors and Affiliations
Contributions
OS was involved in conception and design, acquisition of data, analysis and interpretation of data, drafting the article, and final approval of the version to be published. CF contributed to conception and design, analysis and interpretation of data, drafting the article, and final approval of the version to be published. BV contributed to conception and design, revising the article, and final approval of the version to be published. YM, OS, and CLH were involved in conception and design, analysis and interpretation of data, revising the article, and final approval of the version to be published. KM contributed to acquisition, analysis, and interpretation of the data; revising the article; and final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no relevant conflicts of interest.
Ethical Approval
This work was performed in adherence to ethical guidelines and was approved by Emory University IRB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12028_2019_906_MOESM3_ESM.jpg
Sensitivity analysis. To investigate how sensitive the model coefficients are to different training data sets through a fivefold cross-validation (CV) study. Data were split into fivefold, and each time, we treat onefold as the testing data, train the model on the rest fourfold, and plot the model coefficients. Ideally, the model coefficients should be consistent, meaning the model is stable. For simplicity, the cross-validation results for average consecutive absolute change percentage (ACACP, Panel A) and median consecutive absolute change percentage (MCACP, Panel B) measures. As seen from the plot, the model coefficients are very consistent on each cross-validation fold, indicating the multiple regression models are not sensitive to the training data. At the same time, we can tell the three most influential predictors are the glucose variability, Hunt and Hess grade, and treatment (JPEG 1017 kb)
Rights and permissions
About this article
Cite this article
Sadan, O., Feng, C., Vidakovic, B. et al. Glucose Variability as Measured by Inter-measurement Percentage Change is Predictive of In-patient Mortality in Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 33, 458–467 (2020). https://doi.org/10.1007/s12028-019-00906-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00906-1