Abstract
Background
Cerebral metabolic perturbations are common in aneurysmal subarachnoid hemorrhage (aSAH). Monitoring cerebral metabolism with intracerebral microdialysis (CMD) allows early detection of secondary injury and may guide decisions on neurocritical care interventions, affecting outcome. However, CMD is a regional measuring technique that is influenced by proximity to focal lesions. Continuous microdialysis of the cerebral venous drainage may provide information on global cerebral metabolism relevant for the care of aSAH patients. This observational study aimed to explore the feasibility of jugular bulb microdialysis (JBMD) in aSAH and describe the output characteristics in relation to conventional multimodal monitoring.
Methods
Patients with severe aSAH were included at admission or after in-house deterioration when local clinical guidelines prompted extended multimodal monitoring. Non-dominant frontal CMD, intracranial pressure (ICP), partial brain tissue oxygenation pressure (PbtO2), and cerebral perfusion pressure (CPP) were recorded every hour. The dominant jugular vein was accessed by retrograde insertion of a microdialysis catheter with the tip placed in the jugular bulb under ultrasound guidance. Glucose, lactate, pyruvate, lactate/pyruvate ratio, glycerol, and glutamate were studied for correlation to intracranial measurements. Modified Rankin scale was assessed at 6 months.
Results
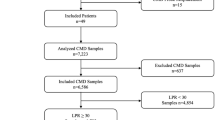
Twelve adult aSAH patients were monitored during a mean 4.2 ± 2.6 days yielding 22,041 data points for analysis. No complications related to JBMD were observed. Moderate or strong significant monotonic CMD-to-JBMD correlations were observed most often for glucose (7 patients), followed by lactate (5 patients), and pyruvate, glycerol, and glutamate (3 patients). Moderate correlation for lactate/pyruvate ratio was only seen in one patient. Analysis of critical periods defined by ICP > 20, CPP < 65, or PbtO2 < 15 revealed a tendency toward stronger CMD-to-JBMD associations in patients with many or long critical periods. Possible time lags between CMD and JBMD measurements were only identified in 6 out of 60 patient variables. With the exception of pyruvate, a dichotomized outcome was associated with similar metabolite patterns in JBMD and CMD. A nonsignificant tendency toward greater differences between outcome groups was seen in JBMD.
Conclusions
Continuous microdialysis monitoring of the cerebral drainage in the jugular bulb is feasible and safe. JBMD-to-CMD correlation is influenced by the type of metabolite measured, with glucose and lactate displaying the strongest associations. JBMD lactate correlated more often than CMD lactate to CPP, implying utility for detection of global cerebral metabolic perturbations. Studies comparing JBMD to other global measures of cerebral metabolism, e.g., PET CT or Xenon CT, are warranted.



Similar content being viewed by others
Abbreviations
- aSAH:
-
Aneurysmal subarachnoid hemorrhage
- CMD:
-
Cerebral microdialysis
- JBMD:
-
Jugular bulb microdialysis
- LPR:
-
Lactate/pyruvate ratio
- ICP:
-
Intracranial pressure
- MAP:
-
Mean arterial pressure
- CPP:
-
Cerebral perfusion pressure
- PbtO2 :
-
Brain tissue oxygenation
- BBB:
-
Blood–brain barrier
- NCC:
-
Neurocritical care
References
Van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. The Lancet. 2007;369:306–18.
Hutchinson PJ, Jalloh I, Helmy A, Carpenter KLH, Rostami E, Bellander B-M, et al. Consensus statement from the 2014 International Microdialysis Forum. Intensive Care Med. 2015;41:1517–28.
Nordström C-H, Koskinen L-O, Olivecrona M. Aspects on the physiological and biochemical foundations of neurocritical care. Front Neurol. 2017;8:274.
Carpenter KLH, Young AMH, Hutchinson PJ. Advanced monitoring in traumatic brain injury: microdialysis. Curr Opin Crit Care. 2017;23:103–9.
Raboel PH, Bartek J, Andresen M, Bellander BM, Romner B. Intracranial pressure monitoring: invasive versus non-invasive methods—a review. Crit Care Res Pract. 2012;2012:950393.
Helbok R, Kofler M, Schiefecker AJ, Gaasch M, Rass V, Pfausler B, et al. Clinical use of cerebral microdialysis in patients with aneurysmal subarachnoid hemorrhage—state of the art. Front Neurol. 2017;8:565.
Engström M, Polito A, Reinstrup P, Romner B, Ryding E, Ungerstedt U, et al. Intracerebral microdialysis in severe brain trauma: the importance of catheter location. J Neurosurg. 2005;102:460–9.
Jakobsen R, Nielsen TH, Granfeldt A, Toft P, Nordström C-H. A technique for continuous bedside monitoring of global cerebral energy state. Intensive Care Med Exp. 2016;4(1):3.
Mølstrøm S, Nielsen TH, Andersen C, Nordström CH, Toft P. Bedside monitoring of cerebral energy state during cardiac surgery—a novel approach utilizing intravenous microdialysis. J Cardiothorac Vasc Anesth. 2017;31:1166–73.
Cormio M, Robertson CS. Ultrasound is a reliable method for determining jugular bulb dominance. J Neurosurg Anesthesiol. 2001;13:250–4.
Gouëzel C, Lorne E, Bonnet V, Fradin S, Saplacan V, Gérard J-L, et al. Assessment of changes in lactate concentration with intravascular microdialysis during high-risk cardiac surgery using the trend interchangeability method. Br J Anaesth. 2017;119:1110–7.
McGreevy DT, Dogan S, Oscarsson V, Vergari M, Eliasson K, Hörer TM, et al. Metabolic response to claudication in peripheral arterial disease: a microdialysis pilot study. Ann Vasc Surg. 2019;58:134–41.
Möller F, Liska J, Eidhagen F, Franco-Cereceda A. Intravascular microdialysis as a method for measuring glucose and lactate during and after cardiac surgery. J Diabetes Sci Technol. 2011;5:1099–107.
Verbeeck RK. Blood microdialysis in pharmacokinetic and drug metabolism studies. Adv Drug Deliv Rev. 2000;45:217–28.
Smith QR. Transport of glutamate and other amino acids at the blood–brain barrier. J Nutr. 2000;130:1016S–22S.
Vejledninger: Dansk Neurokirurgisk Selskab—DNKS. http://dnks.dk/index.php?id=16. Cited 20 Feb 2019.
Newell DW, Winn HR. Transcranial Doppler in cerebral vasospasm. Neurosurg Clin N Am. 1990;1:319–28.
Blixt C, Rooyackers O, Isaksson B, Wernerman J. Continuous on-line glucose measurement by microdialysis in a central vein. A pilot study. Crit Care. 2013;17:R87.
Thelin EP, Nelson DW, Ghatan PH, Bellander B-M. Microdialysis monitoring of CSF parameters in severe traumatic brain injury patients: a novel approach. Front Neurol. 2014;5:159.
Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18:91–3.
Landriel Ibañez FA, Hem S, Ajler P, Vecchi E, Ciraolo C, Baccanelli M, et al. A new classification of complications in neurosurgery. World Neurosurg. 2011;75:709–15.
Reinstrup P, Ståhl N, Mellergård P, Uski T, Ungerstedt U, Nordström C-H. Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery. 2000;47:701–10.
Taccone FS, Badenes R, Arib S, Rubulotta F, Mirek S, Franchi F, et al. Cerebrospinal fluid glucose and lactate levels after subarachnoid hemorrhage: a multicenter retrospective study. J Neurosurg Anesthesiol. 2019. https://doi.org/10.1097/ANA.0000000000000584.
Rostami E, Bellander B-M. Monitoring of glucose in brain, adipose tissue, and peripheral blood in patients with traumatic brain injury: a microdialysis study. J Diabetes Sci Technol. 2011;5:596–604.
Hutchinson PJ, O’Connell MT, Al-Rawi PG, Maskell LB, Kett-White R, Gupta AK, et al. Clinical cerebral microdialysis: a methodological study. J Neurosurg. 2000;93:37–43.
Nielsen TH, Schalén W, Ståhl N, Toft P, Reinstrup P, Nordström CH. Bedside diagnosis of mitochondrial dysfunction after malignant middle cerebral artery infarction. Neurocrit Care. 2014;21:35–42.
Forsse A, Nielsen TH, Nygaard KH, Nordström C-H, Gramsbergen JB, Poulsen FR. Cyclosporin A ameliorates cerebral oxidative metabolism and infarct size in the endothelin-1 rat model of transient cerebral ischaemia. Sci Rep. 2019;9:3702.
Rostami E, Engquist H, Howells T, Johnson U, Ronne-Engström E, Nilsson P, et al. Early low cerebral blood flow and high cerebral lactate: prediction of delayed cerebral ischemia in subarachnoid hemorrhage. J Neurosurg. 2018;128:1762–70.
Helbok R, Schmidt JM, Kurtz P, Hanafy KA, Fernandez L, Stuart RM, et al. Systemic glucose and brain energy metabolism after subarachnoid hemorrhage. Neurocrit Care. 2010;12:317–23.
Jalloh I, Helmy A, Howe DJ, Shannon RJ, Grice P, Mason A, et al. A comparison of oxidative lactate metabolism in traumatically injured brain and control brain. J Neurotrauma. 2018;35:2025–35.
Sahuquillo J, Merino M-A, Sánchez-Guerrero A, Arikan F, Vidal-Jorge M, Martínez-Valverde T, et al. Lactate and the lactate-to-pyruvate molar ratio cannot be used as independent biomarkers for monitoring brain energetic metabolism: a microdialysis study in patients with traumatic brain injuries. PLoS ONE. 2014;9:e102540.
Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32:1107–38.
Hillered L, Valtysson J, Enblad P, Persson L. Interstitial glycerol as a marker for membrane phospholipid degradation in the acutely injured human brain. J Neurol Neurosurg Psychiatry. 1998;64:486–91.
Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3:748–55.
Hawkins RA, Viña JR. How glutamate is managed by the blood–brain barrier. Biology. 2016;5(4):37.
Carteron L, Bouzat P, Oddo M. Cerebral microdialysis monitoring to improve individualized neurointensive care therapy: an update of recent clinical data. Front Neurol. 2017;8:601.
Unterberg AW, Sakowitz OW, Sarrafzadeh AS. Role of bedside microdialysis in the diagnosis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2001;94(5):740–9.
Vajkoczy P, Horn P, Thome C, Munch E, Schmiedek P. Regional cerebral blood flow monitoring in the diagnosis of delayed ischemia following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;98:1227–34.
Sarrafzadeh AS, Haux D, Lüdemann L, Amthauer H, Plotkin M, Küchler I, et al. Cerebral ischemia in aneurysmal subarachnoid hemorrhage: a correlative microdialysis-PET study. Stroke. 2004;35:638–43.
Tholance Y, Barcelos GK, Dailler F, Renaud B, Marinesco S, Perret-Liaudet A. Biochemical neuromonitoring of poor-grade aneurysmal subarachnoid hemorrhage: comparative analysis of metabolic events detected by cerebral microdialysis and by retrograde jugular vein catheterization. Neurol Res. 2015;37:578–87.
Poca MA, Sahuquillo J, Vilalta A, Garnacho A. Lack of utility of arteriojugular venous differences of lactate as a reliable indicator of increased brain anaerobic metabolism in traumatic brain injury. J Neurosurg. 2007;106:530–7.
Pérez A, Minces PG, Schnitzler EJ, Agosta GE, Medina SAP, Ciraolo CA. Jugular venous oxygen saturation or arteriovenous difference of lactate content and outcome in children with severe traumatic brain injury. Pediatr Crit Care Med. 2003;4:33–8.
Alten J, Mariscalco MM. Critical appraisal of Perez et al: Jugular venous oxygen saturation or arteriovenous difference of lactate content and outcome in children with severe traumatic brain injury. Pediatr Crit Care Med. 2005;6:480–2.
Arshi B, Mack WJ, Emanuel B. Invasive and noninvasive multimodal bedside monitoring in subarachnoid hemorrhage: a review of techniques and available data. Neurol Res Int. 2013;2013:987934.
Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. Stroke. 2012;43:1711–37.
Hutchinson PJ, Gupta AK, Fryer TF, Al-Rawi PG, Chatfield DA, Coles JP, et al. Correlation between cerebral blood flow, substrate delivery, and metabolism in head injury: a combined microdialysis and triple oxygen positron emission tomography study. J Cereb Blood Flow Metab. 2002;22:735–45.
Rostami E, Engquist H, Howells T, Ronne-Engström E, Nilsson P, Hillered LT, et al. The correlation between cerebral blood flow measured by bedside Xenon-CT and brain chemistry monitored by microdialysis in the acute phase following subarachnoid hemorrhage. Front Neurol. 2017;8:369.
Enblad P, Valtysson J, Andersson J, Lilja A, Valind S, Antoni G, et al. Simultaneous intracerebral microdialysis and positron emission tomography in the detection of ischemia in patients with subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1996;16:637–44.
Vespa P, Bergsneider M, Hattori N, Wu H-M, Huang S-C, Martin NA, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25:763–74.
Beards SC, Yule S, Kassner A, Jackson A. Anatomical variation of cerebral venous drainage: the theoretical effect on jugular bulb blood samples. Anaesthesia. 1998;53:627–33.
Acknowledgements
The authors thank the staff at the neurointensive care ward at Odense University Hospital for excellent assistance during data collection. We thank Ulrich Halekoh for statistical consults and Claire Gudex for language editing.
Funding
The Syddansk Universitet (Grant No. Department of clinical research faculty scholarship), Odense Universitetshospital (Grant No. PhD scholarship), Karen S Jensens legat (Grant No. 40-A1922), Læge Else Poulsens Mindelegat (Grant No. 53-A2626), Fonden til Lægevidenskabens Fremme (Grant No. 12-260), and Fonden for neurologisk forskning (Grant No. A1281).
Author information
Authors and Affiliations
Contributions
CHN, THN, and AF contributed to conceptualization. THN, CHN, AF, and FRP provided methodology. AF, KSN, and JH performed formal analysis. AF, KHN, SY, and SM were involved in data acquisition. AF wrote the original draft. All authors reviewed and edited the manuscript. FRP and THN supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval/Informed Consent
The study was approved by the Ethical Committee of Southern Denmark under Project No. S-20150173 and by the Danish Data Protection Agency under Project No. 16/36969. Written informed consent has been obtained from all participants or next of kin and a trial guardian.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Forsse, A., Nielsen, T.H., Mølstrøm, S. et al. A Prospective Observational Feasibility Study of Jugular Bulb Microdialysis in Subarachnoid Hemorrhage. Neurocrit Care 33, 241–255 (2020). https://doi.org/10.1007/s12028-019-00888-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00888-0