Abstract
Background
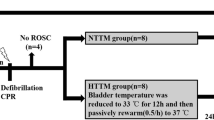
Our previous study found that mild hypothermia (MH) after resuscitation reduced cerebral microcirculation, but the mechanism was not elucidated. The aim of this study was to clarify changes of endothelin-1 (ET-1) and nitric oxide (NO) systems in brain tissue during hypothermia after resuscitation.
Methods
Twenty-six domestic male Beijing Landrace pigs were used in this study. MH was intravascularly induced 1 h after resuscitation from 8-min ventricular fibrillation. Core temperature was reduced to 33 °C and maintained until 8 h after resuscitation, and then animals were euthanized. ET-1 and NO levels in brain tissue and peripheral plasma were measured. Expression of endothelin-converting enzyme-1 (ECE-1), endothelin A receptor (ET-AR), endothelin-B receptor, and nitric oxide synthase (NOS) in brain tissue was determined by Western blot analysis.
Results
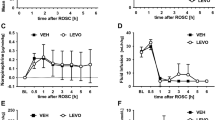
Compared with non-hypothermia (NH) treatment, MH after resuscitation significantly increased the level of endothelin-1 and reduced the level of NO in peripheral blood and brain tissue. Cerebral expression of ECE-1 and ET-AR was significantly increased during MH after resuscitation. Moreover, MH significantly decreased inducible NOS expression compared with the NH group.
Conclusions
The ET-1 system is activated, while inducible NOS is inhibited in brain tissue during MH after resuscitation.






Similar content being viewed by others
References
Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–87.
Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–202.
Neumar RW, Shuster M, Callaway CW, et al. Part 1: executive summary: 2015 american heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S315–67.
Donnino MW, Andersen LW, Berg KM, et al. Temperature management after cardiac arrest: an advisory statement by the advanced life support task force of the international liaison committee on resuscitation and the American Heart Association Emergency Cardiovascular Care Committee and the council on cardiopulmonary, critical care, perioperative and resuscitation. Resuscitation. 2016;98:97–104.
Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197–206.
Wu J, Yuan W, Li J, et al. Effects of mild hypothermia on cerebral large and small microvessels blood flow in a porcine model of cardiac arrest. Neurocrit Care. 2017;27:297–303.
Molenaar P, O’Reilly G, Sharkey A, et al. Characterization and localization of endothelin receptor subtypes in the human atrioventricular conducting system and myocardium. Circ Res. 1993;72:526–38.
Lake EMR, Bazzigaluppi P, Mester J, et al. Neurovascular unit remodelling in the subacute stage of stroke recovery. Neuroimage. 2017;146:869–82.
Idris AH, Becker LB, Ornato JP, et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a task force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Institute of Critical Care Medicine, the Safar Center for Resuscitation Research, and the Society for Academic Emergency Medicine. Resuscitation. 1996;33:69–84.
Wu JY, Li CS, Liu ZX, Wu CJ, Zhang GC. A comparison of 2 types of chest compressions in a porcine model of cardiac arrest. Am J Emerg Med. 2009;27:823–9.
Matsui T, Yoshida Y, Yanagihara M, Suenaga H. Hypothermia at 35 degrees C reduces the time-dependent microglial production of pro-inflammatory and anti-inflammatory factors that mediate neuronal cell death. Neurocrit Care. 2014;20:301–10.
Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S465–82.
Seule M, Muroi C, Sikorski C, Hugelshofer M, Winkler K, Keller E. Therapeutic hypothermia reduces middle cerebral artery flow velocity in patients with severe aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2014;20:255–62.
Donadello K, Favory R, Salgado-Ribeiro D, et al. Sublingual and muscular microcirculatory alterations after cardiac arrest: a pilot study. Resuscitation. 2011;82:690–5.
van Genderen ME, Lima A, Akkerhuis M, Bakker J, van Bommel J. Persistent peripheral and microcirculatory perfusion alterations after out-of-hospital cardiac arrest are associated with poor survival. Crit Care Med. 2012;40:2287–94.
Hodges GJ, Ferguson SAH, Cheung SS. Glabrous and non-glabrous vascular responses to mild hypothermia. Microvasc Res. 2019;121:82–6.
Del Pozzi AT, Carter SJ, Collins AB, Hodges GJ. The regional differences in the contribution of nitric oxide synthase to skin blood flow at forearm and lower leg sites in response to local skin warming. Microvasc Res. 2013;90:106–11.
Del Pozzi AT, Hodges GJ. Comparison of the noradrenergic sympathetic nerve contribution during local skin heating at forearm and leg sites in humans. Eur J Appl Physiol. 2015;115:1155–64.
Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol. 2005;288:H1573–9.
Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol. 2007;292:H1700–5.
Wu J, Wang S, Li C. Hemodynamic and catecholamine changes after recurrent ventricular fibrillation. J Emerg Med. 2013;44:543–9.
Schwarzl M, Steendijk P, Huber S, et al. The induction of mild hypothermia improves systolic function of the resuscitated porcine heart at no further sympathetic activation. Acta Physiol (Oxf). 2011;203:409–18.
Haynes WG, Hamer DW, Robertson CE, Webb DJ. Plasma endothelin following cardiac arrest: differences between survivors and non-survivors. Resuscitation. 1994;27:117–22.
Zoerner F, Wiklund L, Miclescu A, Martijn C. Therapeutic hypothermia activates the endothelin and nitric oxide systems after cardiac arrest in a pig model of cardiopulmonary resuscitation. PLoS ONE. 2013;8:e64792.
Stewart DJ, Kubac G, Costello KB, Cernacek P. Increased plasma endothelin-1 in the early hours of acute myocardial infarction. J Am Coll Cardiol. 1991;18:38–43.
Kolettis TM. Endothelin-1 during myocardial ischaemia: a double-edged sword? Hypertens Res. 2011;34:170–2.
Miyauchi Y, Jesmin S, Sakai S, et al. Effects of selective endothelin (ET)-A receptor antagonist versus dual ET-A/B receptor antagonist on hearts of streptozotocin-treated diabetic rats. Life Sci. 2014;111:6–11.
Phillips L, Toledo AH, Lopez-Neblina F, Anaya-Prado R, Toledo-Pereyra LH. Nitric oxide mechanism of protection in ischemia and reperfusion injury. J Invest Surg. 2009;22:46–55.
Ally A, Maher TJ. Transient middle cerebral artery occlusion and reperfusion alters inducible NOS expression within the ventrolateral medulla and modulates cardiovascular function during static exercise. Can J Physiol Pharmacol. 2011;89:639–46.
Wu D, Bassuk J, Arias J, et al. Different roles of nitric oxide synthase isoforms in cardiopulmonary resuscitation in pigs. Resuscitation. 2007;73:144–53.
Shango DN, Hachimi-Idrissi S, Ebinger G, Michotte Y, Huyghens L. Therapeutic hypothermia preserves the brain by reducing nitric oxide synthase after asphyxial cardiac arrest in rats. Crit Care. 2008;12:375.
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63.
Gong P, Hua R, Zhang Y, et al. Hypothermia-induced neuroprotection is associated with reduced mitochondrial membrane permeability in a swine model of cardiac arrest. J Cereb Blood Flow Metab. 2013;33:928–34.
Howes D, Gray SH, Brooks SC, et al. Canadian guidelines for the use of targeted temperature management (therapeutic hypothermia) after cardiac arrest: a joint statement from The Canadian Critical Care Society (CCCS), Canadian Neurocritical Care Society (CNCCS), and the Canadian Critical Care Trials Group (CCCTG). Resuscitation. 2016;98:48–63.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81801882) and Foundation for Training Excellent Talents of Beijing (No. 2017000021469G219). We thank Liwen Bianji, Edanz Group, China, for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
CL and JW designed the experiment protocol, and reviewed the final manuscript. JW took part in the animal experiment, analyzed experimental data and drafted the manuscript. ZW-L helped draft the manuscript. WY, YZ, JL, ZH-L and JB-L took part in the animal experiment and interpreted the results.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Ethical Approval
This study conformed to ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, J., Li, Z., Yuan, W. et al. Changes of Endothelin-1 and Nitric Oxide Systems in Brain Tissue During Mild Hypothermia in a Porcine Model of Cardiac Arrest. Neurocrit Care 33, 73–81 (2020). https://doi.org/10.1007/s12028-019-00855-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00855-9