Abstract
Background
There is a lack of data describing the risk factors for extubation failure (EF) or tracheostomy placement in pediatric neurocritical care (NCC) patients.
Methods
A retrospective chart review of children admitted to the pediatric intensive care unit who were intubated for >24 h with an acute neurocritical illness and had an extubation attempt. Bivariate and multivariate statistical analysis was performed to determine significant associations of demographic, neurologic, pulmonary, and clinical variables with EF and tracheostomy placement. Analysis of predictive factors for EF (within 48 h) and tracheostomy placement during the hospitalization was conducted on a first extubation attempt group (n = 193) and a second attempt group (n = 23) who experienced either EF or a “late re-intubation” (>48 h–7 days).
Results
Traumatic brain injury (37.3%) and seizures/status epilepticus (31.4%) were the most common diagnoses with neuromuscular weakness patients having the highest risk for EF and tracheostomy placement. EF occurred in 20/193 (10.4%) patients after their first attempt and 6/23 (26.1%) after a second attempt. Compared to those with a fair/strong cough, patients with a weak/absent cough had a relative risk (RR) of 9.4 for EF (95% CI, 4.9–17.9, p < 0.001) and 6.7 (95% CI, 2.3–18.9, p = 0.01) for tracheostomy placement on the first and second attempts, respectively. Glasgow Coma Score (GCS), endotracheal tube (ETT) secretion characteristics, and pulmonary variables were not associated with EF or tracheostomy placement.
Conclusions
A weak/absent cough reflex is associated with an increased risk of failing extubation and placement of a tracheostomy in intubated pediatric NCC patients.
Similar content being viewed by others
Introduction
Although essential in the care of critically ill children, mechanical ventilation (MV) is associated with adverse physiologic effects and complications in 24–40% of pediatric patients [1, 2]. Studies have attempted to better define predictors for extubation failure (EF) in pediatric respiratory failure, but neurologic-based variables have received less attention. With neurocritical illness, in addition to primary pulmonary dysfunction, a patient may require intubation for a low level of consciousness, loss of protective airway reflexes, poor respiratory effort, or ineffective lower airway clearance [3].
An artificial airway is associated with the requirement of sedative medications in young children, the potential for airway injury and it provides a direct conduit for contamination of the tracheobronchial tree with oropharyngeal and gastric flora increasing the risk for ventilator-associated pneumonia (VAP). In injured children there is a 10-fold higher chance for developing pneumonia after three days on MV and a 23-fold higher risk after five days [4] emphasizing the risk of serious complications increase with the duration of MV [5]. Therefore, extubation should be performed as soon as the patient is ready to breath without ventilator support to decrease the complications associated with MV. Although earlier extubation may be advantageous, compared with successfully extubated patients, those who experience EF are at increased risk of mortality, infectious complications, longer durations of MV, and longer hospital stays [6,7,8,9].
While testing of pulmonary function with a spontaneous breathing trial (SBT) prior to extubation is widely practiced in clinical care, extubation success in neurocritical care (NCC) patients may depend on other factors, such as the underlying brain injury, level of consciousness, presence of adequate airway tone, and the ability to cough and clear secretions. Although clinicians frequently consider the neurologic status of the patient, the cough reflex, and secretion characteristics in the extubation decision, these variables have not been previously studied in pediatric patients [10,11,12]. Faced with this uncertainty clinicians may unnecessarily delay extubation in the brain-injured patient due to concerns of altered sensorium [13].
Understanding the factors associated with EF or success specific to pediatric NCC patients could potentially allow for an earlier extubation strategy reducing the duration and complications associated with MV while limiting the physiologic stress and complications associated with re-intubation. Additionally, describing the incidence, demographics and factors that are associated with tracheostomy placement in this population may improve our decision algorithm for considering tracheostomy placement. In this retrospective study, we sought to describe the incidence and risk factors for EF and tracheostomy placement in a cohort of pediatric NCC patients who were intubated in association with the onset of neurologic dysfunction for >24 h and had an attempt at extubation. In addition to studying conventional measures of EF, we wished to examine neurologic-based variables such as the neurologic examination and cough reflex that are reported to have improved ability to predict EF in brain-injured and NCC patients [14,15,16]. Finally, EF is a clinical term used to define re-insertion of an endotracheal tube (ETT) in the first 24–72 h after extubation; we felt it was also important to examine extubation outcomes beyond this period by assessing which patients ultimately received tracheostomies, in-hospital mortality, and re-intubations between 48 h and 7 days.
Materials and Methods
Study Design and Patients
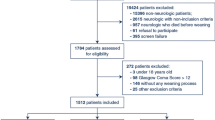
The study received institutional review board approval from UT Southwestern Medical Center and Children’s Medical Center, Dallas. For this type of retrospective study, formal consent was not required. A Crystal Report (SAP Walldorf, Germany) of pre-defined neurologic diagnostic categories was generated using International Classification of Diseases, Ninth Revision, and Clinical Modification (ICD-9) codes from the electronic medical record (EMR) from January 1, 2009, to December 31, 2012. Children, aged 0–18 years, mechanically ventilated with an ETT for ≥24 h with at least one extubation attempt were included. Exclusion criteria were the following, pre-existing tracheostomy, incomplete data, a non-neurologic reason for intubation, extubation in the process of limitation of care, or no extubation attempt (Fig. 1). Although some patients may have been intubated secondary to therapies received as part of their management, for example, after administering seizure medications for status epilepticus, our approach excluded those patients with prior neurologic conditions that were intubated primarily for respiratory or cardiovascular reasons. We included all patients with neuromuscular weakness in our analysis because we believed that neuromuscular weakness itself would be a significant contributor to the reason for intubation. EF was defined as re-insertion of an ETT ≤48 h of extubation. The term “success” referred to patients not requiring re-intubation at 48 h; thus, a patient could have been re-intubated or received a tracheostomy after 48 h and could have been labeled a success. Patients re-intubated from 48 h to 7 days after extubation are referred to as “late re-intubations.”
Extubation Readiness Test and Post-Extubation Therapies
An extubation readiness test (ERT) was the institutional method to evaluate eligibility for extubation. This test, ordered at the medical team’s discretion, consisted of a 1-h pressure support trial of 6–10 cm H2O (depending on ETT size) with a Fi02 of 0.5 and a positive end-expiratory pressure of 5 cm H2O. The patient passed if they maintained a SpO2 >95%, a tidal volume of ≥5 mL/kg, and a respiratory rate within an age-based goal and were extubated in the following 24–48 h based on the treating medical team’s opinion. The decision to perform a tracheostomy or institute noninvasive positive pressure ventilation (NIPPV) (as a bridge or rescue therapy) was also at the discretion of the medical team’s discretion. An ETT leak test was not regularly charted in the EMR for study patients and was not included.
Cough Reflex and Secretion Characteristics
The strength or force of the cough reflex was assessed at the bedside by a respiratory therapist (RT) during a spontaneous or induced cough maneuver and recorded in the EMR as strong, fair, weak, or absent. The cough reflex was categorized as a binary variable (weak/absent vs. fair/strong) for statistical analysis. A weak/absent cough represented the absence or inadequate activation of expiratory intercostal and abdominal muscles during a cough maneuver and inability to expel ETT secretions into the ETT. A fair/strong cough reflex represented normal to moderate activation of abdominal and intercostal muscles during the cough and ability to expel ETT secretions into the ETT. Similarly, the RT recorded secretion amount as large, moderate, or small and consistency as thick or thin in the EMR flow sheet. Small secretions required a single pass with the inline catheter (<5 s) to clear. Moderate or large secretions required multiple passes through the inline catheter for >5 s to clear. Secretion amount was categorized as a binary variable (small vs. moderate/large) for analysis.
Data Collection and Statistical Analysis
A single investigator reviewed each chart to collect pre-extubation data, clinical information, post-extubation therapies, and extubation outcomes. Data were collected from the EPIC flow sheets documentation using the intensive care unit (ICU) FreqVS/Asmt, Ventilator, and Treatment categorical field tabs entered by the nurse or RT as part of routine clinical care. The last recorded variable prior to extubation was used for analysis, typically 1–2 h prior to extubation. Bivariate and multiple logistic modeling was performed on independent variables against EF or extubation “success” as the dependent outcome (Table 1). In addition, demographic data, cough reflex, secretion characteristics, and NIPPV use <48 h were analyzed with tracheostomy placement as the outcome. The State Behavioral Scale (SBS) assessed the child’s level of sedation (−3 = unresponsive, −2 = responsive to noxious stimuli, −1 = responsive to gentle touch or voice, 0 = awake and able to calm, +1 = restless and difficult to calm, and +2 = agitated) [17]. Shapiro–Wilk method was used to test normality of the continuous variables. Normally distributed data were presented as mean and ± standard deviation, and two-sample t test was used for comparisons between two groups. Non-normally distributed data were presented as median and interquartile range (IQR), and Wilcoxon rank-sum test was used to compare two groups. Chi-square test and Fisher’s exact test were used to test association between two categorical variables. Decision tree analysis was used to best collapse diagnostic categories based on their corresponding proportion of re-intubation. Data were analyzed using SAS 9.4 and SAS Enterprise Miner 14.1. Statistical significance level is set at p ≤ 0.05. Stepwise method (entering p value = 0.2 and staying p value = 0.05) and the model fit statistics Akaika’s Information Criteria (AIC) were used to build multiple logistic models for EF.
Results
Study Patients and Pre-extubation Variable Associations with Extubation Failure
EF occurred in 20/193 (10.4%) patients after the first extubation attempt. In patients who remained extubated at 48 h, 7/173 (4%) experienced a late re-intubation and 3/173 (1.7%) who were not re-intubated in the 7 days following extubation went on to have tracheostomies for persistent stridor/airway compromise. A flowchart summarizing the extubation outcomes of study patients after their first and second extubation attempts is presented in Fig. 1. The median age was younger in patients with EF (21 months, IQR [16–66] versus 48 months, IQR [19–108]) although this did not reach statistical significance (p = 0.12). The median hospital length of stay (LOS) (26.5 days, IQR [17.5–44] versus 18 days, IQR [11–28]; p = 0.006) and pediatric intensive care unit (PICU) LOS (14.2 days, IQR [9.2–23.1] versus 8.5 days, IQR [4.3–13.8]; p = 0.003) were both significantly longer in patients experiencing EF (Table 1). The most common diagnoses were traumatic brain injury (TBI) (37.3%) and seizures/status epilepticus (31.6%) (Table 2). In bivariate analysis, the cough reflex was the only significant variable associated with EF (p < 0.001) and an increased heart rate was marginally significant in patients with EF (p = 0.06). No other variables reached statistical significance (Table 1). A weak/absent cough had a relative risk (RR) of 9.4 (95% CI, 4.9–17.9; p < 0.001) for EF with a positive predictive value (PPV) of 0.8 and a negative predictive value (NPV) of 0.91. The level of sedation as assessed by the SBS or use of sedatives within 12 h of extubation did not differ between those experiencing EF and those successfully extubated. Only 2/193 (1%) patients died that had an extubation attempt: one from oncologic disease and the second was withdraw of support due to a terminal illness.
Decision tree analysis was performed to explore EF risk within diagnostic categories. Patients with the neuromuscular weakness were found to have an increased risk of EF compared to other diagnoses (Table 2). In multiple logistic model building, stepwise method showed that patients with weak/absent cough reflex had a higher risk of EF (p = 0.001). The model fit statistics AIC was used as an alternative method to build multiple logistic model. Both cough reflex and age were selected as the predictors for EF. In this model, patients with weak/absent cough reflex (p = 0.001) and younger age had a higher risk of EF (p = 0.13).
Second Extubation Attempt
The second extubation attempt group included 16 patients with EF and seven late re-intubations for a total of 23 patients (Fig. 1). Although those with “late failures” did not meet the definition of EF, we included these patients in a second attempt group as they ultimately required re-insertion of an ETT and extubation was attempted a second time. EF occurred in 6/23 (26.1%) of second attempt patients. There were no variables statistically associated with EF in the second attempt group (Supplemental Table 1). While the association of study variables with EF did not reach significance in this group, we might have found significant associations with a larger sample size. For example, patients with EF overall were younger (median = 20.5 months, IQR [16–44]) and had longer durations of intubation (median = 7.5 days, IQR [3.5–12.7]) compared to success patients (median = 48 months, IQR [16–132]) and duration of intubation (median = 3.5 days, IQR [2.5–7.0]).
Reasons for Extubation Failure and Post-Extubation Therapies
Patients failed extubation (n = 26) for the following reasons: upper airway obstruction/stridor (38.5%), hypoxemia without stridor (23.1%), hypoventilation without stridor (3.8%), apnea/poor effort (7.7%), inability to handle secretions (11.5%), seizure (3.8%), increased work of breathing (3.8%), or unable to determine (7.7%). There were no associations with the use of racemic epinephrine, steroid, or heliox therapy with extubation outcome (Table 3). The use of NIPPV within 48 h of extubation was higher in the second attempt group 8/23 (34.8%) compared to the first attempt group 19/193 (16%), but its use was not significant with respect to extubation outcome. It is worth noting though that in the first group only 3/19 (15.7%) of children who were placed on NIPPV <48 h had EF compared to 4/4 (66.7%) with the second attempt. NIPPV was used as rescue therapy in 10/19 (52.3%) and 5/8 (62.5%) for the first and second attempt groups, respectively, with the remainder being extubated directly to NIPPV.
Tracheostomy Placement in Pediatric Neurocritical Care Patients
The tracheostomy rate was 9/193 (4.7%), 3 in the first attempt and 6 in the second attempt group. There were no predictors for tracheostomy placement in the first attempt group. A pre-extubation weak/absent cough was associated with a RR of 6.7 (95% CI, 2.3–18.9; p = 0.01) for tracheostomy placement in the second attempt group with a PPV of 1.0 and a NPV of 0.85. We also found in the second attempt group that NIPPV use <48 h conferred a increased risk of tracheostomy placement, RR = 9.4 (95% C, 1.3–67.167.1; p < 0.01). Clinicians favoring re-intubation and a second attempt may in part explain why a weak/absent cough reflex was associated with EF but not tracheostomy placement in the first attempt group. Sixteen patients received tracheostomies that did not meet study eligibility. Descriptive statistics for all tracheostomy patients (n = 25) and for patients who received a tracheostomy with or without an extubation attempt are presented in Table 4. Patients with hypoxic-ischemic brain injury (28%) and neuromuscular weakness (36%) represented a much higher percentage of children who received tracheostomies in comparison to their frequency in total sample population. Patients with hypoxic-ischemic injury were more likely to have a tracheostomy placed without an extubation attempt (7/7) compared to patients with neuromuscular disease.
Discussion
Pediatric NCC patients are at increased risk of mortality and PICU stays compared with the general PICU population [18, 19]. Thirty-six percent of NCC patients will have co-existing medical conditions and infectious complications due to lower respiratory tract infections can occur in 40% of children after severe TBI and cardiac arrest [20, 21]. Notably, the VAP risk in pediatric TBI patients (12.2 per 1000 ventilator days) is significantly higher than the general PICU population (2.9 per 1000 ventilator days) [22].
To decrease the complications associated with invasive MV, extubation should be performed as soon as a patient is able to breathe without MV assistance. The majority of children will have a SBT prior to extubation, but in brain-injured patients the ETT may also function to counteract poor glottic or pharyngeal tone or facilitate suctioning of tracheal and bronchial secretions in patients with impaired airway clearance. In addition to assessing the patient’s pre-extubation status, the immediate post-extubation care in the 12–24 h following extubation is critically important to avoid re-intubation. There are no studies to date examining variables of extubation outcome that are specific to neurologic critical illness in children. We found that a weak/absent pre-extubation cough reflex was the most significant risk factor for predicting both EF and tracheostomy placement in pediatric NCC patients and that the neurologic status assessed by GCS and conventional parameters of extubation readiness did not accurately predict EF risk. We also found that patients with neuromuscular weakness represented the highest diagnostic category risk for EF and for receiving a tracheostomy and if a pediatric NCC patient survived to an extubation attempt their in-hospital mortality was very low.
Incidence and Risk Factors for Extubation Failure in Pediatric Neurocritical Illness
Although there is wide variability in the published rate of EF in pediatric patients ranging from 5.0 to 16.3%, the mean rate is estimated to be roughly 6% [6, 23,24,25]. We report an EF rate of 10.4% within 48 h and 14% within 7 days of extubation in pediatric NCC patients mechanically ventilated for >24 h. Patients with EF required an additional 6 days of MV and 10 more hospital days compared to successfully extubated patients.
There is clinical variability and a lack of consensus on the optimal timing of extubation in a child with neurocritical illness, and clinicians may delay extubation due to concerns of altered sensorium potentially increasing the risk for associated complications. In a study by Namen et al., a median of two days passed in 82% of brain-injured patients after passing a SBT prior to an extubation attempt [14]. Coplin et al. also reported in a study of children and adults that comatose patients with extubation delay (>48 h after passing an SBT) had a 3.7-fold increase in the incidence of pneumonia as compared to comatose patients extubated within 48 h of passing a SBT [13]. The high mean pre-extubation GCS we observed would imply that clinicians in general waited until the level of neurologic arousal improved prior to extubation.
There are valid concerns that premature extubation of a neurologically impaired patient could potentially increase morbidity but controversy exist on the association of the GCS with EF and what level of neurologic function is sufficient for extubation success [26]. Some authors have argued that the ability to follow commands is more predictive than the GCS for EF [15, 27]. We found no association with the pre-extubation GCS and EF. It is interesting to note that in our study there were 38 patients who were extubated with a GCS < 8 and only one experienced EF. Additionally, only 1/8 children with a documented unplanned extubation required reintubation. The effect that the level on consciousness by itself has on EF warrants further study but it may be that some children can be safely extubated even with lower GCS. The level of consciousness may be relevant in the context to other variables such as, timing from insult, secretion management, airway edema, cough reflex strength, and degree of neuromuscular weakness. Although we did not report significant associations of many of the variables with EF, due to the small sample size a larger prospective study including multiple factors is needed to support our findings.
In medical ICU patients after passing a SBT, a weak or absent cough conferred a fourfold increase in risk of EF [28]. In another study, a voluntary cough peak flow rate of <60 L/min was associated with a RR of 4.8 for EF [15]. In agreement with these studies we report that patients with a weak/absent cough had a significant increase in the risk for EF which remained significant in multiple variable modeling. While we did not find any significant associations with post-extubation therapies such as NIPPV use or racemic epinephrine treatments, care was not protocolized and we did not examine all aspect of post-extubation care that may have influenced the extubation outcome. Establishing more objective measures of the cough reflex and including this variables in the pre-extubation planning might lead to a more targeted post-extubation care plan and reduce EF. For example, if a patient was found to have a cough reflex measure that would predict a high risk for EF, they could potentially be managed with a standardized approach of airway clearance and cough assist therapies in the period immediately post-extubation.
Tracheostomy Placement in Pediatric NCC Patients
Clinicians may appropriately take a cautious approach to tracheostomy placement in children after neurologic injury since many will experience significant neurologic improvement during their hospitalization. Nevertheless, controversy exists whether earlier tracheostomy would be beneficial in some patients and criteria are not well established when this should occur. In a retrospective study by Holscher et al., tracheostomy <7 days post-injury after pediatric TBI was associated with a decrease in ventilator days, ICU, and hospital days and airway complications compared with late tracheostomy >7 days post-injury [29]. There are many variables used in the clinical decision process of when to perform a tracheostomy, which we could not control for. In addition, there were no established protocols or guidelines to establish selection criteria for tracheostomy patients. Given this limitation we report that a weak/absent cough reflex was a strong predictor after a previously failed extubation attempt for receiving a tracheostomy. In fact, all three patients with a weak/absent cough in the second attempt group were re-intubated and received tracheostomies. While the sample size was small for a second attempt population, consideration for tracheostomy placement might be reasonable in patients who still exhibit a weak or absent cough reflex after an previous failed attempt. Analysis of the sub group of patients who received tracheostomies revealed that patients with neuromuscular disease and hypoxic–ischemic injury were the patients most likely to receive a tracheostomy and we found no difference in the hospital or PICU LOS in patients who had an extubation attempt followed by tracheostomy versus those without an attempt (Table 4). Interestingly, although TBI represented 37.3% of the total sample population, it comprised only 4% of the patients who received a tracheostomy.
Although EF is associated with increased mortality in adult critical care patients, evidence in pediatrics is limited. Farias et al. reported in-unit mortality (39.3 vs. 2.9%) and in-hospital mortality (46.4 vs. 6.3%) were significantly higher for general PICU patients with EF [30]. Kurachek et al. also reported a higher mortality in children with EF although none of the deaths were temporally related to extubation [6]. After excluding patients where extubation was part of the withdrawal process or death occurred before an extubation attempt, only 2 children died; neither of them experienced EF. This suggests that despite serious neurologic injury, if a child survives to an extubation attempt, their in-hospital mortality is very low.
Study Strengths and Limitations
The study strengths include the large number of pediatric NCC patients and inclusion of neurologic-based variables in this analysis of EF. However, there are also several limitations to the study. A major limitation is the lack of an objective measure for the cough reflex which was the main variable associated with extubation outcome. While the results were dichotomized into weak/absent and strong/fair categories, using more objective measures of cough reflex and secretion characteristics would improve the study’s conclusions. Variables related to infection or neuromuscular blockade were not evaluated. In addition, the retrospective nature of the study limited variables to those that could be objectively scored from the EMR. Because the decision for performing a ERT was determined by the attending physician, this could have influenced the prevalence of EF; therefore, it is possible there is high center-to-center variability in the EF rate predictive values. In addition, there were no institutional protocol-driven pathways for sedation, ventilator weaning, post-extubation care, or pulmonary toilet, e.g., chest physiotherapy or cough assist therapy during the study period, which could have influenced the results.
Conclusions
The decision to extubate is of considerable consequence as both delayed extubation and EF are associated with the potential to increase patient morbidity. In this retrospective study, extubation failure occurred in 10.4% of NCC patients and younger age and a weak/absent cough reflex were assocaited with an increased risk for EF. A weak/absent cough reflex conferred a ninefold increase in risk of EF in patients on their first attempt and sixfold increase in the risk for receiving a tracheostomy after a previous failed extubation. Conventional weaning parameters, sedation scores and the GCS were not associated with extubation outcome. Patients with neuromuscular weakness had the highest risk of EF and represented the largest percentage of patients who received tracheostomies compared to other NCC diagnostic categories. This study adds to our knowledge of risk factors for EF and tracheostomy placement in children with neurocritical illness and acute respiratory failure.
References
Rivera R, Tibballs J. Complications of endotracheal intubation and mechanical ventilation in infants and children. Crit Care Med. 1992;20(2):193–9.
Principi T, Fraser DD, Morrison GC, Farsi SA, Carrelas JF, Maurice EA, Kornecki A. Complications of mechanical ventilation in the pediatric population. Pediatr Pulmonol. 2011;46(5):452–7.
Harel Y, Vardi A, Quigley R, Brink LW, Manning SC, Carmody TJ, Levin DL. Extubation failure due to post-extubation stridor is better correlated with neurologic impairment than with upper airway lesions in critically ill pediatric patients. Int J Pediatr Otorhinolaryngol. 1997;39(2):147–58.
Ortega HW, Cutler G, Dreyfus J, Flood A, Kharbanda A. Hospital-acquired pneumonia among pediatric trauma patients treated at national trauma centers. J Trauma Acute Care Surg. 2015;78(6):1149–54.
Monteverde E, Fernandez A, Poterala R, Vidal N, Siaba Serrate A, Castelani P, Albano L, Podesta F, Farias JA. Characterization of pediatric patients receiving prolonged mechanical ventilation. Pediatr Crit Care Med. 2011;12(6):e287–91.
Kurachek SC, Newth CJ, Quasney MW, Rice T, Sachdeva RC, Patel NR, Takano J, Easterling L, Scanlon M, Musa N, et al. Extubation failure in pediatric intensive care: a multiple-center study of risk factors and outcomes. Crit Care Med. 2003;31(11):2657–64.
Elward AM, Warren DK, Fraser VJ. Ventilator-associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes. Pediatrics. 2002;109(5):758–64.
Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112(1):186–92.
Farias JA, Alia I, Retta A, Olazarri F, Fernandez A, Esteban A, Palacios K, Di Nunzio L, Fernandez G, Bordon A, et al. An evaluation of extubation failure predictors in mechanically ventilated infants and children. Intensiv Care Med. 2002;28(6):752–7.
Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324(21):1445–50.
Manczur TI, Greenough A, Pryor D, Rafferty GF. Comparison of predictors of extubation from mechanical ventilation in children. Pediatr Crit Care Med. 2000;1(1):28–32.
Baumeister BL, El-Khatib M, Smith PG, Blumer JL. Evaluation of predictors of weaning from mechanical ventilation in pediatric patients. Pediatr Pulmonol. 1997;24(5):344–52.
Coplin WM, Pierson DJ, Cooley KD, Newell DW, Rubenfeld GD. Implications of extubation delay in brain-injured patients meeting standard weaning criteria. Am J Respir Crit Care Med. 2000;161(5):1530–6.
Namen AM, Ely EW, Tatter SB, Case LD, Lucia MA, Smith A, Landry S, Wilson JA, Glazier SS, Branch CL, et al. Predictors of successful extubation in neurosurgical patients. Am J Respir Crit Care Med. 2001;163(3 Pt 1):658–64.
Salam A, Tilluckdharry L, Amoateng-Adjepong Y, Manthous CA. Neurologic status, cough, secretions and extubation outcomes. Intensiv Care Med. 2004;30(7):1334–9.
Ko R, Ramos L, Chalela JA. Conventional weaning parameters do not predict extubation failure in neurocritical care patients. Neurocrit Care. 2009;10(3):269–73.
Curley MA, Harris SK, Fraser KA, Johnson RA, Arnold JH. State Behavioral Scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006;7(2):107–14.
Bell MJ, Carpenter J, Au AK, Keating RF, Myseros JS, Yaun A, Weinstein S. Development of a pediatric neurocritical care service. Neurocrit Care. 2009;10(1):4–10.
Wainwright MS, Grimason M, Goldstein J, Smith CM, Amlie-Lefond C, Revivo G, Noah ZL, Harris ZL, Epstein LG. Building a pediatric neurocritical care program: a multidisciplinary approach to clinical practice and education from the intensive care unit to the outpatient clinic. Semin Pediatr Neurol. 2014;21(4):248–54.
Hutchison JS, Ward RE, Lacroix J, Hebert PC, Barnes MA, Bohn DJ, Dirks PB, Doucette S, Fergusson D, Gottesman R, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23):2447–56.
Cirulis MM, Hamele MT, Stockmann CR, Bennett TD, Bratton SL. Comparison of the new adult ventilator-associated event criteria to the Centers for Disease Control and Prevention Pediatric Ventilator-Associated Pneumonia Definition (PNU2) in a population of pediatric traumatic brain injury patients. Pediatr Crit Care Med. 2016;17(2):157–64.
Alharfi IM, Charyk Stewart T, Al Helali I, Daoud H, Fraser DD. Infection rates, fevers, and associated factors in pediatric severe traumatic brain injury. J Neurotrauma. 2014;31(5):452–8.
Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, Luckett PM, Forbes P, Lilley M, Thompson J, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288(20):2561–8.
Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, Pollack M, Zimmerman J, Anand KJ, Carcillo JA, et al. Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med. 2009;10(1):1–11.
Edmunds S, Weiss I, Harrison R. Extubation failure in a large pediatric ICU population. Chest. 2001;119(3):897–900.
King CS, Moores LK, Epstein SK. Should patients be able to follow commands prior to extubation? Respir Care. 2010;55(1):56–65.
Anderson CD, Bartscher JF, Scripko PD, Biffi A, Chase D, Guanci M, Greer DM. Neurologic examination and extubation outcome in the neurocritical care unit. Neurocrit Care. 2011;15(3):490–7.
Khamiees M, Raju P, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest. 2001;120(4):1262–70.
Holscher CM, Stewart CL, Peltz ED, Burlew CC, Moulton SL, Haenel JB, Bensard DD. Early tracheostomy improves outcomes in severely injured children and adolescents. J Pediatr Surg. 2014;49(4):590–2.
Farias JA, Retta A, Alia I, Olazarri F, Esteban A, Golubicki A, Allende D, Maliarchuk O, Peltzer C, Ratto ME, et al. A comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensiv Care Med. 2001;27(10):1649–54.
Acknowledgements
We would like to especially recognize Andre Finley’s dedication to helping children and his invaluable support to research and the Departments of Children’s Health Clinical Research and Advanced Practice Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cohn, E.C., Robertson, T.S., Scott, S.A. et al. Extubation Failure and Tracheostomy Placement in Children with Acute Neurocritical Illness. Neurocrit Care 28, 83–92 (2018). https://doi.org/10.1007/s12028-017-0429-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-017-0429-0