Abstract
Background
We sought to determine whether therapeutic temperature modulation (TTM) to treat fever after intracerebral hemorrhage (ICH) is associated with improved hospital complications and discharge outcomes.
Methods
We performed a retrospective case–control study of patients admitted with spontaneous ICH having two consecutive fevers ≥38.3 °C despite acetaminophen administration. Cases were enrolled from a prospective database of patients receiving TTM from 2006 to 2010. All cases received TTM for fever control with goal temperature of 37 °C with a shiver-control protocol. Controls were matched in severity by ICH score and retrospectively obtained from 2001 to 2004, before routine use of TTM for ICH. Primary outcome was discharge-modified Rankin score.
Results
Forty patients were enrolled in each group. Median admission ICH Score, ICH volume, and GCS were similar. TTM was initiated with a median of 3 days after ICH onset and for a median duration of 7 days. Mean daily T max was significantly higher in the control group over the first 12 days (38.1 vs. 38.7 °C, p ≤ 0.001). The TTM group had more days of IV sedation (median 8 vs. 1, p < 0.001) and mechanical ventilation (18 vs. 9, p = 0.003), and more frequently underwent tracheostomy (55 vs. 23 %, p = 0.005). Mean NICU length of stay was longer for TTM patients (15 vs. 11 days, p = 0.007). There was no difference in discharge outcomes between the two groups (overall mortality 33 %, moderate or severe disability 67 %).
Conclusions
Therapeutic normothermia is associated with increased duration of sedation, mechanical ventilation, and NICU stay, but is not clearly associated with improved discharge outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous, non-traumatic intracerebral hemorrhage (ICH) is a disease notable not only for its morbidity and mortality, but also for its lack of effective therapies [1]. Despite a number of large randomized-controlled trials focusing on the management of ICH performed in the last decade, there has been no change in the mortality of the disease over a similar timeframe [2]. Although the 2010 AHA/ASA ICH guidelines mention the use of therapeutic temperature modulation (TTM) for fever control as a possible neuroprotective strategy after ICH, no recommendation was made due to lack of outcomes data [3].
The availability and use of TTM technology have increased recently as a result of its use in post-cardiac arrest hypothermia, but there is little data available on the safety and efficacy of TTM for fever control in ICH [4]. TTM is an attractive candidate for fever control in ICH for a number of reasons. First, fever after ICH is common. Approximately one-third of patients with ICH will experience fever, a significant portion of which is not explained by infection and is likely central in origin [5]. Second, early fever has been shown to be an independent predictor of early neurologic deterioration, suggesting that fever itself may cause secondary cerebral injury [6]. Third, the duration of fever has been independently associated with poor outcome in a dose-related response, making reduction of fever burden an attractive therapy to prevent secondary injury [7]. Fourth, TTM has been shown to be feasible and safe in awake patients with ischemic stroke, subarachnoid hemorrhage, and to a limited degree, ICH [8–10].
We performed a case–control study in ICH patients with fever to determine the impact of TTM on fever burden and its association with hospital complications and discharge outcomes.
Materials and Methods
Data Collection and Patient Selection
Cases were selected from the Columbia TTM Database, a prospectively collected database of all patients treated with a TTM device in our Neurological Intensive Care Unit (NICU) since January 2006. Inclusion criteria for cases were: (1) admission to Columbia NICU with diagnosis of ICH visualized by non-contrast head CT; (2) consecutive fevers ≥38.3 °C over 2 h despite the use of acetaminophen; and (3) TTM utilized for fever control with goal temperature of 37 °C. Exclusion criteria included TTM to goal temperature other than 37 °C, death or withdrawal of care within 72 h of admission, and ICH due to trauma, tumor, aneurysm, hemorrhagic conversion of ischemic infarct, or venous thrombosis. Baseline data collection for the Columbia TTM Database included demographics, as well as admission clinical and laboratory data. Upon initiation of TTM, data collection included daily temperature goal, minimum and maximum temperature, best GCS score, routine daily chemistries, a tabulated SIRS score, and dose of vasoactive and sedative medications. Scoring of infectious complications using standard criteria for pneumonia, urinary tract infection, meningitis/ventriculitis, bloodstream infection, and Clostridium difficile infection as well as antibiotic use was recorded daily.
Controls were selected from the retrospective portion of the Columbia Intracerebral Hemorrhage Outcomes Project (ICHOP), a database of all patients with spontaneous ICH admitted to the Columbia NICU. Controls were screened from the years 2001 to 2004, which corresponds to the time period immediately before our routine use of TTM for fever control in patients with ICH. Inclusion criteria for controls were admission to Columbia NICU between January 2001 and December 2004 with admission diagnosis of ICH visualized by non-contrast head CT and two consecutive hourly fevers ≥38.3 °C despite the use of acetaminophen. Exclusion criteria included the use of TTM, death, or withdrawal of care within 72 h of admission, and ICH due to trauma, tumor, aneurysm, hemorrhagic conversion of ischemic infarct, or venous thrombosis. Controls were then matched to cases based on ICH score (11). Demographic, radiographic, and clinical course data were retrospectively collected.
ICH size was calculated in a blinded manner using the ABC/2 method on the Columbia NICU admission CT scan. Outcome data for all patients were interpreted from written examinations from neurologist or physical therapy notes by an author trained in evaluation of modified Rankin scores.
The Columbia University Institutional Review Board has approved the conduct of both the Columbia TTM Database and ICHOP and waived the need for informed consent.
Fever Management and Therapeutic Temperature Modulation Protocol
All patients with fever ≥38.3 °C received acetaminophen (650 mg every 4–6 h orally) and a water-circulating blanket (Blanketrol II). For cases, an institutional guideline was established for the uniform application of TTM. This guideline stipulated that TTM was used only in patients who, after initially being treated with acetaminophen and a water-circulating blanket, had a persistent fever for at least 2 h. TTM was initiated with a surface TTM device (Arctic Sun; Medivance, Inc., Louisville, CO, USA) with target temperature of 37 °C. The duration of therapy was decided by the clinical neurocritical care team on a case-by-case basis. Temperature was recorded by a bladder temperature probe. Patients receiving TTM were placed on a shiver-prevention regimen which has been previously described [11].
Intracerebral Hemorrhage Treatment Protocol
All patients were admitted to the CUMC NICU. Initial management of patients with ICH at our institution involves rapid reduction in systolic blood pressure to <160 with use of either nicardipine or labetalol drips. Patient with coagulopathy received FFP and 3 days of 10 mg vitamin K to maintain INR < 1.5. Patient on platelet inhibitors received one 0.3 mcg/kg IV dose of desmopressin and one unit (six packs) of platelets. Patients with uremia received a similar dose of desmopressin. Most patients received a vascular imaging study within the first 72 h by either CT, MR, or conventional angiography. Surgery was reserved for patients with mass effect significant enough to warrant hemicraniectomy as determined by the attending neurosurgeon on-service. External ventricular drains (EVDs) were placed for patients with hydrocephalus, extensive IVH, or suspected intracranial hypertension. Controls were placed on hypertonic saline solutions to target sodium values to 145–155. Due to change in treatment protocol, cases did not receive empiric hypertonic saline solutions and rather received bolus osmotherapy for elevation in ICP > 20 cm H2O (mannitol 1 g/kg or 30 cc sodium chloride 23.4 % solution). Treatment of casted IVH with intrathecal tPA was performed on a case-by-case basis as determined by clinical attending.
Statistical Analysis
Baseline characteristics and in-hospital complications were recorded as categorical or continuous variables. All categorical variables are reported as a proportion and percentile and were analyzed using χ 2 or Fisher’s exact test. Normally distributed continuous variables are reported as a mean (± standard deviation) and analyzed using Student’s t test. Non-normally distributed variables are reported as medians (interquartile range) and analyzed using the Mann–Whitney U test. A post hoc power analysis demonstrated an ability to detect an absolute difference in good outcome (mRS 0–3) of 30 % given 40 patients in each group.
Results
Baseline Characteristics
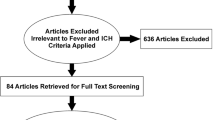
Forty patients with ICH who received TTM for fever control were enrolled and matched to 40 historical controls by ICH Score (Fig. 1). Baseline characteristics at admission for each group are presented in Table 1. Cases and controls were evenly matched for ICH score, but baseline GCS trended slightly lower in the TTM group compared to controls (6 vs. 8, p = 0.08). There were also more non-white patients in the control group (60 vs. 80 %, p = 0.05).
Comparison of Daily Maximum Temperature
Figure 2 shows the median daily maximum temperature and the percentage of febrile patients in each group. Admission temperatures were not different between the two groups (37.1 vs. 37.7 °C, p = 0.07). TTM was started on a median bleed day of 2.5 (IQR 1–4.5) and median days of TTM was 6 (IQR 3–9). All patients received cooling with a surface cooling device. For the 21 patients with at least one recorded Bedside Shivering Assessment Scale (BSAS), the average BSAS was 0.8 and 50 % of values 1 or higher (shivering present).
Medical Complications
Table 2 summarizes medical complications in the two groups. Patients receiving TTM were more likely to be intubated, have more days of mechanical ventilation and sedation, have longer NICU stays, and to undergo tracheostomy. Hyperglycemia was also more common in patients receiving TTM (93 vs. 60 %, p = 0.001). There were also more ventilator-associated pneumonias and DVTs in the TTM group, but these did not reach statistical significance.
Outcomes
There was no measurable difference in discharge outcomes between groups (mRS 4–5 65 % in each group, p = 1.0). In-hospital mortality and rates of transitioning to comfort measures were similar in the two groups (Table 3).
Risk Factors for Tracheostomy Placement
Given the increased rates of tracheostomy placement, we tested the association of tracheostomy placement with other clinical variables. Categorical variables associated with tracheostomy placement included receiving TTM (p = 0.01), receiving hemicraniectomy (p = 0.03), presence of hydrocephalus on admission scan (p = 0.04), peak glucose >180 (p = 0.005), and more than 6 days of TTM (0.05). When placed into a binomial logistic regression model for tracheostomy placement, TTM use for more than 6 days (OR 6.1, 95 % CI 1.7–21.7), hemicraniectomy (OR 5.1, 95 % CI 1.3–20.0), and hydrocephalus on admission (OR 3.3, 95 % CI 1.1–10.1) were associated with tracheostomy.
During the years of 2005–2006, the neurointensive care physicians at our institution started performing percutaneous tracheostomies, a procedure previously performed by the ENT consult service. Given the possible influence of this change of practice on rates of tracheostomy, we looked at tracheostomy rates in a separate group of patients in our NICU to see if there was a difference in tracheostomy rates in the two time periods. We analyzed the rates of tracheostomy in intubated, poor-grade (Hunt-Hess Grade 4–5) subarachnoid hemorrhage patients during the same time periods as the current study (excluding those who received TTM) and found similar rates in the two periods (17.6 % in the 2001–2004 epoch vs. 13.1 % of patients in the 2006–2010 epoch).
Discussion
While one previous study has examined the role of prophylactic normothermia in patients with brain injury including ICH, to the best of our knowledge this is the first publication examining the use of TTM for normothermia in patients with fever specifically after ICH. Patients receiving TTM had longer NICU lengths of stay, more days of mechanical ventilation and sedation, and higher rates of tracheostomy placement compared to those receiving conventional fever control. We were unable to demonstrate a difference in discharge functional outcomes or mortality between the two groups, though the study was only powered to detect a large absolute difference.
While fever has consistently been demonstrated to be an independent predictor of poor outcome after ICH, it remains unclear if reduction of fever burden is clinically meaningful. Our group previously demonstrated a small benefit in 12 months functional outcomes for febrile SAH patients receiving TTM for fever control [12]. In that study, however, we were unable to demonstrate a measurable benefit on functional outcomes at discharge and 3 months, calling into the question the small benefit at 12 months. While this current study does not answer the question regarding utility of fever control, it does indicate that under our current practice of TTM for fever control, the benefit—if any—is modest and the risks are real and measurable.
We hypothesize that the increased length of mechanical ventilation and higher rates of tracheostomy are related to the sedation often needed for shiver control during ICH. Unfortunately, bedside assessments of shivering (BSAS) were not consistently performed during the early years of this study and thus an accurate description of rates of shivering is not available. While shiver control is thought to be essential for the benefit of TTM, improvement in the BSAS often comes via higher levels of sedation. While shivering is thought to counteract some of the potential benefit of fever control, any potential neuroprotective effect of normothermia may also be negated by the medical complications of sedation. Different strategies of fever control need to be explored to better examine this question. The analysis on intubated, high-grade SAH patients during the same time periods gives us confidence that the increased rate of tracheostomy in the TTM group of this study does not appear to be part of a general trend in our unit toward more tracheostomies and is likely related to increased sedative use. We also do not feel that the increased rates of intubation and days of mechanical ventilation are part of a shift in the aggressiveness of our treatment, as rates of withdrawal of care were similar between groups.
One prior study by Broessner et al. [8] examined the use of prophylactic, endovascular normothermia in patients with either poor-grade SAH, large ischemic stroke, or ICH with GCS < 10 versus conventional fever therapy. Their study, of which 40 % were ICH patients, found no significant difference in overall rates of adverse events between normothermia and control groups, but there was a significant increase in infectious complications with normothermia (96 % in normothermia vs. 78 %, p = 0.04). In all patients (individual ICH data not available for any outcomes), they found similar length of NICU stay, as well as rates of death and disability at discharge, day 30, and 6 months in both groups. Although they report no difference in major adverse events, some of the major findings of our study, increased rates of sedation, intubation and tracheostomy, were not reported in their study. The treatment in shivering was different in the two studies, namely sole use of meperidine in the Broessner study and a step-wise approach including dexmedetomidine and propofol in our current study [11]. This may explain some differences and should be an area of future study. Overall, the studies both failed to show a significant treatment effect of normothermia.
There are several weaknesses to this study. It is underpowered to detect a moderate or even large effect of TTM for normothermia on outcomes in this population. The single-center design makes generalizability of the findings more difficult, and the retrospective collection of controls makes all findings less robust than a prospective study. The lack of long-term outcome data is an unfortunate aspect of the retrospective case–control design. Despite an extensive search, long-term records (3 and 6 months post-ictus) were not available for the vast majority of patients in the control group. Although lack of long-term follow-up is a significant weakness, prior studies of TTM in cardiac arrest demonstrate that the significant portion of benefit in mortality is apparent on discharge [4]. While we agree that there are significant limitations to the study, we feel that the significant findings should be reported.
Conclusion
At some centers, including ours, fever control with TTM has become the “standard of care” without clear demonstration of its benefit. This study, while not answering the question of whether fever control may be neuroprotective, helps to establish clinical equipoise and potential risks to its implementation and larger, randomized trials are needed before further widespread adoption.
References
Rincon F, Mayer SA. Clinical review: critical care management of spontaneous intracerebral hemorrhage. Crit Care. 2008;12(6):237.
Andaluz N, Zuccarello M. Recent trends in the treatment of spontaneous intracerebral hemorrhage: analysis of a nationwide inpatient database. J Neurosurg. 2009;110(3):403–10.
Morgenstern LB, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–29.
The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56.
Commichau C, Scarmeas N, Mayer SA. Risk factors for fever in the neurologic intensive care unit. Neurology. 2003;60(5):837–41.
Leira R, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63(3):461–7.
Schwarz S, et al. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54(2):354–61.
Broessner G, et al. Influence of red blood cell transfusion on mortality and long-term functional outcome in 292 patients with spontaneous subarachnoid hemorrhage. Crit Care Med. 2009;37(6):1886–92.
Guluma KZ, et al. A trial of therapeutic hypothermia via endovascular approach in awake patients with acute ischemic stroke: methodology. Acad Emerg Med. 2006;13(8):820–7.
Kammersgaard LP, et al. Feasibility and safety of inducing modest hypothermia in awake patients with acute stroke through surface cooling: a case–control study: the Copenhagen Stroke Study. Stroke. 2000;31(9):2251–6.
Choi HA, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care. 2011;14(3):389–94.
Badjatia N, et al. Impact of induced normothermia on outcome after subarachnoid hemorrhage: a case–control study. Neurosurgery. 2010;66(4):696–700 discussion 700–1.
Acknowledgments
Stephan Mayer has received consulting fees from Acetlion, Biogen Idec, CSL Behring, Haemonetics, Medivance/CR Bard, Neuroptics, Orsan Technologies, Pfizer, Sage Therapeutics, Sanofi-Aventis, Stryker, and Edge Therapeutics; Stock/Stock Options in Orsan Technologies.
Conflict of interest
Aaron S. Lord, Sarah Karinja, Hector Lantigua, Amanda Carpenter, J. Michael Schmidt, Jan Claassen, Sachin Agarwal, E. Sander Connolly, and Neeraj Badjatia declare no conflicts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lord, A.S., Karinja, S., Lantigua, H. et al. Therapeutic Temperature Modulation for Fever After Intracerebral Hemorrhage. Neurocrit Care 21, 200–206 (2014). https://doi.org/10.1007/s12028-013-9948-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-013-9948-5