Abstract
Aim
Hyperammonemia causes brain edema and high intracranial pressure (ICP) in acute liver failure (ALF) by accumulation of glutamine in brain. Since a high-level glutamine may compromise mitochondrial function, the aim of this study was to determine if the lactate–pyruvate ratio is associated with a rise in the glutamine concentration and ICP.
Patients and Methods
In 13 patients with ALF (8F/5M; median age 46 (range 18–66) years) the cerebral extracellular concentrations of glutamine, lactate, and pyruvate were measured by in vivo brain microdialysis together with ICP and cerebral perfusion pressure (CPP).
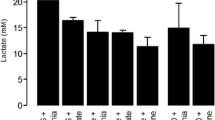
Results
The cerebral glutamine concentration was 4,396 (1,011–9,712) μM, lactate 2.15 (1.1–4.45) mM, and pyruvate 101 (43–255) μM. The lactate–pyruvate ratio was 21 (16–40), ICP 20 (2–28) mmHg, and CPP 72 (56–115) mmHg. Cerebral glutamine concentration correlated with the lactate–pyruvate ratio (r = 0.89, P < 0.05). Also the ICP, but not CPP, correlated to the lactate–pyruvate ratio (r = 0.64, P < 0.05).
Conclusion
ICP and the cerebral glutamine concentration in patients with ALF correlate to the lactate–pyruvate ratio. Since CPP was sufficient in all patients the rise in lactate–pyruvate ratio indicates that accumulation of glutamine compromises mitochondrial function and causes intracranial hypertension.

Similar content being viewed by others
Abbreviations
- ALF:
-
Acute liver failure
- ALT:
-
Alanine aminotransferase
- BBB:
-
Blood–brain barrier
- CPP:
-
Cerebral perfusion pressure
- HE:
-
Hepatic encephalopathy
- ICP:
-
Intracranial pressure
- INR:
-
International Normalization Ratio
- MPT:
-
Mitochondrial permeability transition
- NE:
-
Norepinephrine
- PaCO2 :
-
Carbon dioxide tension in arterial blood
- ROS:
-
Reactive oxygen species
References
O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet 1993;342(8866):273–5.
Takahashi H, Koehler RC, Brusilow SW, Traystman RJ. Inhibition of brain glutamine accumulation prevents cerebral edema in hyperammonemic rats. Am J Physiol 1991;261(3 Pt 2):H825–9.
Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology 1999;29(3):648–53.
Strauss GI, Knudsen GM, Kondrup J, Moller K, Larsen FS. Cerebral metabolism of ammonia and amino acids in patients with fulminant hepatic failure. Gastroenterology 2001;121(5):1109–19.
Bhatia V, Singh R, Acharya SK. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut 2006;55(1):98–104.
Ott P, Larsen FS. Blood-brain barrier permeability to ammonia in liver failure: a critical reappraisal. Neurochem Int 2004;44(4):185–98.
Pedersen HR, Ring-Larsen H, Olsen NV, Larsen FS. Hyperammonemia acts synergistically with lipopolysaccharide in inducing changes in cerebral hemodynamics in rats anaesthetised with pentobarbital. J Hepatol 2007;47(2):245–52.
Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science 1977;195(4284):1356–8.
Chatauret N, Zwingmann C, Rose C, Leibfritz D, Butterworth RF. Effects of hypothermia on brain glucose metabolism in acute liver failure: a H/C-nuclear magnetic resonance study. Gastroenterology 2003;125(3):815–24.
Brusilow SW, Traystman R. Hepatic encephalopathy. N Engl J Med 1986;314(12):786–7.
Tofteng F, Hauerberg J, Hansen BA, Pedersen CB, Jorgensen L, Larsen FS. Persistent arterial hyperammonemia increases the concentration of glutamine and alanine in the brain and correlates with intracranial pressure in patients with fulminant hepatic failure. J Cereb Blood Flow Metab 2006;26(1):21–7.
Norenberg MD. Oxidative and nitrosative stress in ammonia neurotoxicity. Hepatology 2003;37(2):245–8.
Albrecht J, Norenberg MD. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology 2006;44(4):788–94.
Jayakumar AR, Rama Rao KV, Murthy ChRK, Norenberg MD. Glutamine in the mechanism of ammonia-induced astrocyte swelling. Neurochem Int 2006;48(6–7):623–8.
Murphy N, Auzinger G, Bernel W, Wendon J. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology 2004;39(2):464–70.
Haussinger D, Kircheis G, Fischer R, Schliess F, vom Dahl S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema? J Hepatol 2000;32(6):1035–8.
Hilgier W, Olson JE. Brain ion and amino acid contents during edema development in hepatic encephalopathy. J Neurochem 1994;62(1):197–204.
Cordoba J, Gottstein J, Blei AT. Glutamine, myo-inositol, and organic brain osmolytes after portocaval anastomosis in the rat: implications for ammonia-induced brain edema. Hepatology 1996;24(4):919–23.
Schliess F, Gorg B, Haussinger D. Pathogenetic interplay between osmotic and oxidative stress: the hepatic encephalopathy paradigm. Biol Chem 2006;387(10–11):1363–70.
Larsen FS. Optimal management of patients with fulminant hepatic failure: targeting the brain. Hepatology 2004;39(2):299–301.
Rama Rao KV, Chen M, Simard JM, Norenberg MD. Suppression of ammonia-induced astrocyte swelling by cyclosporin A. J Neurosci Res 2003;74(6):891–7.
Merenda A, Bullock R. Clinical treatments for mitochondrial dysfunctions after brain injury. Curr Opin Crit Care 2006;12(2):90–6.
Kristian T. Metabolic stages, mitochondria and calcium in hypoxic/ischemic brain damage. Cell Calcium 2004;36(3–4):221–33.
Marion DW, Puccio A, Wisniewski SR, Kochanek P, Dixon CE, Bullian L, Carlier P. Effect of hyperventilation on extracellular concentrations of glutamate, lactate, pyruvate, and local cerebral blood flow in patients with severe traumatic brain injury. Crit Care Med 2002;30(12):2619–25.
Hutchinson PJ, O’Connell MT, Al-Rawi PG, Kett-White R, Gupta AK, Kirkpatrick PJ, Pickard JD. Clinical cerebral microdialysis – determining the true extracellular concentration. Acta Neurochir Suppl 2002;81:359–62.
Reinstrup P, Stahl N, Mellergard P, Uski T, Ungerstedt U, Nordstrom CH. Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery 2000;47(3):701–9.
Wright G, Shawcross D, Olde Damink SW, Jalan R. Brain cytokine flux in acute liver failure and its relationship with intracranial hypertension. Metab Brain Dis 2007;22(3–4):375–88.
Dethloff TJ, Knudsen GM, Larsen FS. Cerebral blood flow autoregulation in experimental liver failure. J Cereb Blood Flow Metab. 2007 Dec 5; (epub ahead of print).
Acknowledgments
This work was supported by Rigshospitalet, University of Copenhagen, The Laerdal Foundation for Acute Medicine, Savvaerksejer Jeppe Juhl and wife Ovita Juhls Foundation, The Novo Nordisk Foundation, The AP-Moeller Foundation, The Danish Hospital Foundation for Medical Research – Region of Copenhagen, The Faroe Islands and Greenland and The Danish Medical Association Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bjerring, P.N., Hauerberg, J., Frederiksen, HJ. et al. Cerebral Glutamine Concentration and Lactate–Pyruvate Ratio in Patients with Acute Liver Failure. Neurocrit Care 9, 3–7 (2008). https://doi.org/10.1007/s12028-008-9060-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-008-9060-4