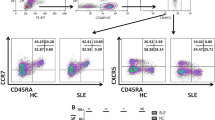
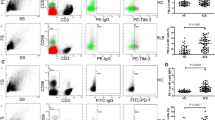
Abstract
This study aims to elucidate the expression and functionality of SIT1 in circulating CD8/CD4 + T cells in humans and to delineate its significance in systemic lupus erythematosus (SLE) patients. We employed multiparametric flow cytometry to investigate the expression of SIT1 in circulating CD8/CD4 + T cells and their respective subsets, comparing healthy controls (HCs) with SLE patients. Furthermore, we assessed the levels of granzyme B, perforin, IL-17, and IFN-γ in SIT1-related CD8/CD4 + T cells from both HCs and SLE patients, both before and after PMA stimulation. Clinically, we conducted receiver operating characteristic curve analysis and correlation analysis to evaluate the clinical relevance of SIT1-related CD8/CD4 + T cells in SLE patients. SIT1 exhibited higher expression in CD4 + T cells, with SIT1 − T cells demonstrating elevated levels of granzyme B, perforin, and IFN-γ compared to SIT1 + T cells. PMA-stimulated T cells exhibited reduced SIT1 expression compared to unstimulated T cells. SLE patients displayed increased SIT1 + proportions in CD8 + T cells and decreased SIT1 + CD4 + T cell numbers. Additionally, SIT1 + cells in SLE patients exhibited significantly higher levels of granzyme B and perforin compared to HCs. SIT1 + cells demonstrated significant associations with clinical indicators in SLE patients, with indicators related to SIT1 proving valuable in the diagnosis of SLE patients. SIT1 is inversely correlated with T cell activation. In SLE patients, SIT1 expression is altered in T cells concomitant with an augmented secretion of cytotoxic molecules. This upregulation may contribute to the pathogenesis of SLE and enhance its diagnostic potential.
Graphical abstract






Similar content being viewed by others
Data availability
The data of this study are available from the corresponding author upon reasonable request.
Abbreviations
- SIT1 :
-
Signaling threshold regulating transmembrane adaptor 1
- SLE :
-
Systemic lupus erythematosus
- HC :
-
Healthy control
- TEM :
-
Effector memory T cell
- TEMRA :
-
Effector memory T cells reexpressing CD45RA
- TCM :
-
Central memory cell
- Tn :
-
Naive T cell
- MFI :
-
Mean fluorescence intensity
- NF-AT :
-
Nuclear factor of T cells
- PBMC :
-
Peripheral blood mononuclear cells
- PKC :
-
Protein kinase C
- PLC :
-
Phospholipase C
- C4 :
-
Complement component 4
- CRP :
-
C-Reactive Protein
- ESR :
-
Erythrocyte sedimentation rate
- FBS :
-
Fetal bovine serum
- GZMB :
-
Granzyme B
- IgA :
-
Immunoglobulin A
- IgG :
-
Immunoglobulin G
- IgM :
-
Immunoglobulin M
- ITIMs :
-
Immunoreceptor tyrosine-based inhibitory motifs
- AUC :
-
Area under the curve
- ROC :
-
Receiver operating characteristic curve
References
Mills JA. Systemic lupus erythematosus. N Engl J Med. 1994;330(26):1871–9.
Kiriakidou M, Ching CL. Systemic Lupus Erythematosus. Ann Intern Med. 2020;172(11):Itc81–96.
Rasaratnam I, Ryan PF. Systemic lupus erythematosus. Med J Aust. 1997;166(5):266–70.
Tian J, et al. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann Rheum Dis. 2023;82(3):351–6.
Li H, et al. Abnormalities of T cells in systemic lupus erythematosus: new insights in pathogenesis and therapeutic strategies. J Autoimmun. 2022;132:102870.
Hedrich CM, et al. cAMP response element modulator α controls IL2 and IL17A expression during CD4 lineage commitment and subset distribution in lupus. Proc Natl Acad Sci U S A. 2012;109(41):16606–11.
Wong CK, et al. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127(3):385–93.
Marie-Cardine A, et al. SHP2-interacting transmembrane adaptor protein (SIT), a novel disulfide-linked dimer regulating human T cell activation. J Exp Med. 1999;189(8):1181–94.
Pfrepper KI, et al. Structural and functional dissection of the cytoplasmic domain of the transmembrane adaptor protein SIT (SHP2-interacting transmembrane adaptor protein). Eur J Immunol. 2001;31(6):1825–36.
Simeoni L, et al. The transmembrane adapter protein SIT regulates thymic development and peripheral T-cell functions. Mol Cell Biol. 2005;25(17):7557–68.
Arndt B, et al. The transmembrane adaptor protein SIT inhibits TCR-mediated signaling. PLoS ONE. 2011;6(9):e23761.
Wilkinson B, Wang H, Rudd CE. Positive and negative adaptors in T-cell signalling. Immunology. 2004;111(4):368–74.
Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15(6):388–400.
Cao X, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27(4):635–46.
Catalfamo M, Henkart PA. Perforin and the granule exocytosis cytotoxicity pathway. Curr Opin Immunol. 2003;15(5):522–7.
Chen PM, Tsokos GC. The role of CD8+ T-cell systemic lupus erythematosus pathogenesis: an update. Curr Opin Rheumatol. 2021;33(6):586–91.
Xu S, Cao X. Interleukin-17 and its expanding biological functions. Cell Mol Immunol. 2010;7(3):164–74.
Ritzmann F, et al. IL-17 cytokines and chronic lung diseases. Cells. 2022;11(14):2132.
Liu W, Zhang S, Wang J. IFN-γ, should not be ignored in SLE. Front Immunol. 2022;13:954706.
Ciurtin C, et al. CD8+ T-cells in juvenile-onset SLE: from pathogenesis to comorbidities. Front Med (Lausanne). 2022;9:904435.
Tan EM, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7.
Tian Y, et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat Commun. 2017;8(1):1473.
Takahama Y, Nakauchi H. Phorbol ester and calcium ionophore can replace TCR signals that induce positive selection of CD4 T cells. J Immunol. 1996;157(4):1508–13.
Kosalka J, Jakiela B, Musial J. Changes of memory B- and T-cell subsets in lupus nephritis patients. Folia Histochem Cytobiol. 2016;54(1):32–41.
Posevitz V, et al. Regulation of T cell homeostasis by the transmembrane adaptor protein SIT. J Immunol. 2008;180(3):1634–42.
Devarajan P, Chen Z. Autoimmune effector memory T cells: the bad and the good. Immunol Res. 2013;57(1–3):12–22.
Verma K, et al. Human CD8+ CD57- TEMRA cells: Too young to be called “old”. PLoS ONE. 2017;12(5):e0177405.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82271755, 81871230) and Peking University People’s Hospital Scientific Research Development Funds (RZ 2022–06).
Author information
Authors and Affiliations
Contributions
CL took charge of all the work and participated in its design. AB and AH conducted most of the experiments and drafted the manuscript. QL and ZZ analyzed the data. XZ and MZ did part of the cellular experiments. ZY did part of the clinical measurements. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hasimu, A., Bahabayi, A., Xiong, Z. et al. SIT1 identifies circulating hypoactive T cells with elevated cytotoxic molecule secretion in systemic lupus erythematosus patients. Immunol Res (2024). https://doi.org/10.1007/s12026-024-09481-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12026-024-09481-w