Abstract
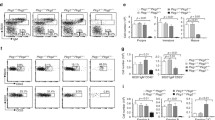
The P4-type ATPases are believed to function as flippases that contribute to the organization of the asymmetric aminophospholipid distribution on the plasma membranes of eukaryotes by their ability to internalize specific phospholipids from the outer leaflet to the inner leaflet. Despite the existence of 14 members of the P4-type ATPases in humans and 15 in mice, their roles in the immune system have not been fully understood. So far, ATP11C was shown to be important for B cells, and mice deficient for ATP11C had a developmental arrest at the pro-B to pre-B cell transition stage of B cell development. Using an ATP11C-deficient pre-B cell line generated through CRISPR/Cas9 engineering, we here tested the role of ATP11C in pre-B cells in vitro and showed that ablation of ATP11C in pre-B cells causes a defect in the flippase activity. We further demonstrated that loss of ATP11C does not impede the proliferation of pre-B cells in response to IL-7. However, pre-B cells lacking ATP11C failed to differentiate into immature B cells upon removal of IL-7. These results suggest that disruption of lipid asymmetry by loss of ATP11C in pre-B cells may control the switch from proliferation to differentiation in pre-B cells.



Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- Ig:
-
Immunoglobulin
- Pre-BCR:
-
Precursor B cell receptor
- IL-7:
-
Interleukin-7
- PS:
-
Phosphatidylserine
- PE:
-
Phosphatidylethanolamine
References
Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26(6):715–25. https://doi.org/10.1016/j.immuni.2007.05.010. (S1074-7613(07)00289-0 [pii]).
Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. https://doi.org/10.1146/annurev.immunol.19.1.595.
Herzog S, Reth M, Jumaa H. Regulation of B-cell proliferation and differentiation by pre-B-cell receptor signalling. Nat Rev Immunol. 2009;9(3):195–205. https://doi.org/10.1038/nri2491. (nri2491 [pii]).
Clark MR, Mandal M, Ochiai K, Singh H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat Rev Immunol. 2014;14(2):69–80. https://doi.org/10.1038/nri3570.
Hendriks RW, Middendorp S. The pre-BCR checkpoint as a cell-autonomous proliferation switch. Trends Immunol. 2004;25(5):249–56. https://doi.org/10.1016/j.it.2004.02.011.
Reth M, Nielsen P. Signaling circuits in early B-cell development. Adv Immunol. 2014;122:129–75. https://doi.org/10.1016/B978-0-12-800267-4.00004-3.
Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350(6317):423–6. https://doi.org/10.1038/350423a0.
Kitamura D, Kudo A, Schaal S, Muller W, Melchers F, Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992;69(5):823–31.
Torres RM, Flaswinkel H, Reth M, Rajewsky K. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science. 1996;272(5269):1804–8.
Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Ig beta. Science. 1996;272(5260):411–4.
Mundt C, Licence S, Shimizu T, Melchers F, Martensson IL. Loss of precursor B cell expansion but not allelic exclusion in VpreB1/VpreB2 double-deficient mice. J Exp Med. 2001;193(4):435–45.
Pelanda R, Braun U, Hobeika E, Nussenzweig MC, Reth M. B cell progenitors are arrested in maturation but have intact VDJ recombination in the absence of Ig-alpha and Ig-beta. J Immunol. 2002;169(2):865–72.
Rolink AG, Winkler T, Melchers F, Andersson J. Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment. J Exp Med. 2000;191(1):23–32.
Chen D, Tang TX, Deng H, Yang XP, Tang ZH. Interleukin-7 biology and its effects on immune cells: mediator of generation, differentiation, survival, and homeostasis. Front Immunol. 2021;12:747324. https://doi.org/10.3389/fimmu.2021.747324.
Samitas K, Lotvall J, Bossios A. B cells: from early development to regulating allergic diseases. Arch Immunol Ther Exp (Warsz). 2010;58(3):209–25. https://doi.org/10.1007/s00005-010-0073-2.
van der Mark VA, Elferink RP, Paulusma CC. P4 ATPases: flippases in health and disease. Int J Mol Sci. 2013;14(4):7897–922. https://doi.org/10.3390/ijms14047897.
Hankins HM, Baldridge RD, Xu P, Graham TR. Role of flippases, scramblases and transfer proteins in phosphatidylserine subcellular distribution. Traffic. 2015;16(1):35–47. https://doi.org/10.1111/tra.12233.
Shin HW, Takatsu H. Phosphatidylserine exposure in living cells. Crit Rev Biochem Mol Biol. 2020;55(2):166–78. https://doi.org/10.1080/10409238.2020.1758624.
Yabas M, Teh CE, Frankenreiter S, Lal D, Roots CM, Whittle B, et al. ATP11C is critical for the internalization of phosphatidylserine and differentiation of B lymphocytes. Nat Immunol. 2011;12(5):441–9. https://doi.org/10.1038/ni.2011. (ni.2011 [pii]).
Siggs OM, Arnold CN, Huber C, Pirie E, Xia Y, Lin P, et al. The P4-type ATPase ATP11C is essential for B lymphopoiesis in adult bone marrow. Nat Immunol. 2011;12(5):434–40. https://doi.org/10.1038/ni.2012. (ni.2012 [pii]).
Yabas M, Jing W, Shafik S, Broer S, Enders A. ATP11C facilitates phospholipid translocation across the plasma membrane of all leukocytes. PLoS One. 2016;11(1):e0146774. https://doi.org/10.1371/journal.pone.0146774.
Yabas M, Coupland LA, Cromer D, Winterberg M, Teoh NC, D’Rozario J, et al. Mice deficient in the putative phospholipid flippase ATP11C exhibit altered erythrocyte shape, anemia, and reduced erythrocyte life span. J Biol Chem. 2014;289(28):19531–7. https://doi.org/10.1074/jbc.C114.570267.
Siggs OM, Schnabl B, Webb B, Beutler B. X-linked cholestasis in mouse due to mutations of the P4-ATPase ATP11C. Proc Natl Acad Sci U S A. 2011;108(19):7890–5. https://doi.org/10.1073/pnas.1104631108. (1104631108 [pii]).
Flemming A, Brummer T, Reth M, Jumaa H. The adaptor protein SLP-65 acts as a tumor suppressor that limits pre-B cell expansion. Nat Immunol. 2003;4(1):38–43. https://doi.org/10.1038/ni862.
Lindner SE, Lohmuller M, Kotkamp B, Schuler F, Knust Z, Villunger A, et al. The miR-15 family reinforces the transition from proliferation to differentiation in pre-B cells. EMBO Rep. 2017;18(9):1604–17. https://doi.org/10.15252/embr.201643735.
Hutter K, Lohmuller M, Jukic A, Eichin F, Avci S, Labi V, et al. SAFB2 Enables the processing of suboptimal stem-loop structures in clustered primary miRNA transcripts. Mol Cell. 2020;78(5):876-89 e6. https://doi.org/10.1016/j.molcel.2020.05.011.
Jensen MS, Costa S, Gunther-Pomorski T, Lopez-Marques RL. Cell-based lipid flippase assay employing fluorescent lipid derivatives. Methods Mol Biol. 2016;1377:371–82. https://doi.org/10.1007/978-1-4939-3179-8_33.
Quah BJ, Parish CR. New and improved methods for measuring lymphocyte proliferation in vitro and in vivo using CFSE-like fluorescent dyes. J Immunol Methods. 2012;379(1–2):1–14. https://doi.org/10.1016/j.jim.2012.02.012.
Jing W, Yabas M, Broer A, Coupland L, Gardiner EE, Enders A, et al. Calpain cleaves phospholipid flippase ATP8A1 during apoptosis in platelets. Blood Adv. 2019;3(3):219–29. https://doi.org/10.1182/bloodadvances.2018023473.
Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344(6188):1164–8. https://doi.org/10.1126/science.1252809.
Takatsu H, Tanaka G, Segawa K, Suzuki J, Nagata S, Nakayama K, et al. Phospholipid flippase activities and substrate specificities of human type IV P-type ATPases localized to the plasma membrane. J Biol Chem. 2014;289(48):33543–56. https://doi.org/10.1074/jbc.M114.593012.
Arashiki N, Takakuwa Y, Mohandas N, Hale J, Yoshida K, Ogura H, et al. ATP11C is a major flippase in human erythrocytes and its defect causes congenital hemolytic anemia. Haematologica. 2016;101(5):559–65. https://doi.org/10.3324/haematol.2016.142273.
Segawa K, Yanagihashi Y, Yamada K, Suzuki C, Uchiyama Y, Nagata S. Phospholipid flippases enable precursor B cells to flee engulfment by macrophages. Proc Natl Acad Sci U S A. 2018;115(48):12212–7. https://doi.org/10.1073/pnas.1814323115.
Milne CD, Paige CJ. IL-7: a key regulator of B lymphopoiesis. Semin Immunol. 2006;18(1):20–30. https://doi.org/10.1016/j.smim.2005.10.003. (S1044-5323(05)00085-0 [pii].).
Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180(5):1955–60.
von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181(4):1519–26.
Yabas M. B cell deficiency and anaemia caused by mutations in the murine Atp11c gene. Canberra, Australia: PhD, The Australian National University; 2014.
Marshall AJ, Fleming HE, Wu GE, Paige CJ. Modulation of the IL-7 dose-response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression. J Immunol. 1998;161(11):6038–45.
Fleming HE, Paige CJ. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity. 2001;15(4):521–31.
Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9(1):93–103. https://doi.org/10.1016/s1074-7613(00)80591-9.
Acknowledgements
We thank Dr. Sebastian Herzog from the Medical University of Innsbruck, Austria, for providing wk3 cells, reagents, and his excellent advice and help during the generation of the ATP11C-deficient pre-B cell line as well as for critical reading of the manuscript. We also thank Michael Lohmüller, Felix Eichin, and Katharina Hutter from the Medical University of Innsbruck, Austria, for sharing their scientific and technical expertise.
Funding
This study was supported by the Trakya University Scientific Research Projects Coordination Unit (Project Number 2019/280).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yabas, M., Bostanci, A. & Aral, S. ATP11C promotes the differentiation of pre-B cells into immature B cells but does not affect their IL-7-dependent proliferation. Immunol Res 71, 609–616 (2023). https://doi.org/10.1007/s12026-023-09364-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-023-09364-6