Abstract
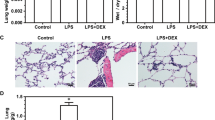
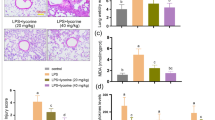
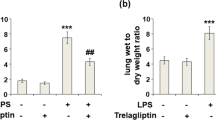
Acute lung injury (ALI) is a severe complication of sepsis and hemorrhagic shock with high morbidity. In the present study, the protective effect of Azilsartan on lipopolysaccharide (LPS)-induced ALI in mice was investigated to explore the potential therapeutic property of Azilsartan for the treatment of ALI. LPS was used to induce an ALI model in mice. Hematoxylin–eosin (HE) staining sections were then evaluated for the pathological state of lung tissues. Bronchoalveolar lavage fluid (BALF) protein concentration, wet/dry weight ratios of lung tissues, and pulmonary myeloperoxidase (MPO) activity were detected to determine the degree of pulmonary injury. The number of total cells, macrophages, and neutrophils in BALF were counted using a hemocytometer to illustrate the inflammatory cell infiltration. The lung function was monitored using a spirometer. The concentrations of interleukin-1β (IL-1β), monocyte chemoattractant protein-1 (MCP-1), and interleukin-8 (IL-8) were determined using enzyme-linked immunosorbent assay (ELISA). Oxidative stress was evaluated by the superoxide dismutase (SOD) activity, glutathione (GSH), and malondialdehyde (MDA) concentrations in the lung tissue. The expressions of nuclear erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) were determined using Western blot analysis. Azilsartan therapy alleviated LPS-induced lung tissue damage, increased BALF protein concentration, lung wet to dry weight ratio, MPO activity, and macrophage and neutrophils infiltration. Also, Azilsartan ameliorated the production of inflammatory factors (IL-1β, MCP-1, and IL-8). Azilsartan ameliorated LPS-impaired lung SOD activity, the GSH concentration, and the MDA concentration. Mechanistically, Azilsartan activated the LPS-impaired Nrf2/HO-1 signaling pathway. Azilsartan therapy attenuates LPS-induced ALI via the Nrf2/HO-1 signaling pathway.








Similar content being viewed by others
Data availability
Experimental data are available on the reasonable request to the corresponding author.
References
Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140:345–50.
Toy P. Update on transfusion-related acute lung injury. Clin Adv Hematol Oncol. 2019;17:378–81.
Bonfanti N, Gundert E, Drewry Goff K, Bedimo R, Kulstad E. Core warming of coronavirus disease 2019 (COVID-19) patients undergoing mechanical ventilation-a protocol for a randomized controlled pilot study. PLoS One. 2020;15:e0243190.
Dhama K, Patel SK, Natesan S, Vora KS, Iqbal Yatoo M, Tiwari R, Saxena SK, Singh KP, Singh R, Malik YS. COVID-19 in the elderly people and advances in vaccination approaches. Hum Vaccin Immunother. 2020;3:1–6.
Beck TN, Young NG, Erickson ML, Prats I. Rare antibody-associated hemolytic transfusion reaction and transfusion-related acute lung injury: a case report. BMC Surg. 2017;17:48.
Karima R, Matsumoto S, Higashi H, Matsushima K. The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today. 1999;5:123–32.
Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–56.
Tasaka S, Amaya F, Hashimoto S, Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal. 2008;10:739–53.
Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–72. https://doi.org/10.1056/NEJMra1608077.
Asti C, Ruggieri V, Porzio S, Chiusaroli R, Melillo G, Caselli GF. Lipopolysaccharide-induced lung injury in mice. I. Concomitant evaluation of inflammatory cells and haemorrhagic lung damage. Pulm Pharmacol Ther. 2000;13:61–9.
Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–99.
Xiao Q, Cui Y, Zhao Y, Liu L, Wang H, Yang L. Orientin relieves lipopolysaccharide-induced acute lung injury in mice: the involvement of its anti-inflammatory and anti-oxidant properties. Int Immunopharmacol. 2021;90:107189. https://doi.org/10.1016/j.intimp.2020.107189.
Amatullah H, Maron-Gutierrez T, Shan Y, Gupta S, Tsoporis JN, Varkouhi AK, Teixeira Monteiro AP, He X, Yin J, Marshall JC, Rocco PRM, Zhang H, Kuebler WM, Dos Santos CC. Protective function of DJ-1/PARK7 in lipopolysaccharide and ventilator-induced acute lung injury. Redox Biol. 2020;38:101796.
Farooq MA, Niazi AK, Akhtar J, Saifullah J, Farooq M, Souri Z, Karimi N, Rengel Z. Acquiring control: the evolution of ROS-induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol Biochem. 2019;141:353–69.
Sindhu S, Akhter N, Kochumon S, Thomas R, Wilson A, Shenouda S, Tuomilehto J, Ahmad R. Increased expression of the innate immune receptor TLR10 in obesity and type-2 diabetes: association with ROS-mediated oxidative stress. Cell Physiol Biochem. 2018;45:572–90.
Tanaka M, Kubot M, Shimoda M, Hayase T, Miyaguchi M, Kobayashi N, Ikeda M, Ishima Y, Kawahara M. Thioredoxin-albumin fusion protein prevents urban aerosol-induced lung injury via suppressing oxidative stress-related neutrophil extracellular trap formation. Environ Pollut. 2020;268:115787.
Turillazzi E, Neri M, Cerretani D, Cantatore S, Frati P, Moltoni L, Busardo FP, Pomara C, Riezzo I, Fineschi V. Lipid peroxidation and apoptotic response in rat brain areas induced by long-term administration of nandrolone: the mutual crosstalk between ROS and NF-kB. J Cell Mol Med. 2016;20:601–12.
Bi XG, Li ML, Xu W, You JY, Xie D, Yuan XF, Xiang Y. Helix B surface peptide protects against acute lung injury through reducing oxidative stress and endoplasmic reticulum stress via activation of Nrf2/HO-1 signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:6919–30.
Huang JL, Fan RZ, Zou YH, Zhang L, Yin S, Tang GH. Salviplenoid A from Salvia plebeia attenuates acute lung inflammation via modulating NF-kappaB and Nrf2 signaling pathways. Phytother Res. 2020. https://doi.org/10.1002/ptr.6922.
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248.
White WB, Weber MA, Sica D, Bakris GL, Perez A, Cao C, Kupfer S. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. 2011;57:413–20.
Kajiya T, Ho CJ, Wang R, Vilard TW. Kurtz, Molecular and cellular effects of azilsartan: a new generation angiotensin II receptor blocker. J Hypertens. 2011;29:2476–83.
de Araujo AA, Varela H, de Medeiros CA, de Castro Brito GA, de Lima KC, de Moura LM, de Araujo Junior RF. Azilsartan reduced TNF-alpha and IL-1beta levels, increased IL-10 levels and upregulated VEGF, FGF, KGF, and TGF-alpha in an oral mucositis model. PLoS One. 2015;10:e0116799.
Hye Khan MA, Neckar J, Haines J, Imig JD. Azilsartan improves glycemic status and reduces kidney damage in zucker diabetic fatty rats. Am J Hypertens. 2014;27:1087–95.
Ma C, Ma Z, Liao XL, Liu J, Fu Q, Ma S. Immunoregulatory effects of glycyrrhizic acid exerts anti-asthmatic effects via modulation of Th1/Th2 cytokines and enhancement of CD4(+)CD25(+)Foxp3+ regulatory T cells in ovalbumin-sensitized mice. J Ethnopharmacol. 2013;148:755–62.
Zhang J, Jiang W, Zuo Z. Pyrrolidine dithiocarbamate attenuates surgery-induced neuroinflammation and cognitive dysfunction possibly via inhibition of nuclear factor kappaB. Neuroscience. 2014;261:1–10.
Chen D, Ma T, Liu XW, Yang C, Liu Z. Rapamycin reverses paraquat-induced acute lung injury in a rat model through inhibition of NFkappaB activation. Int J Clin Exp Pathol. 2015;8:4627–38.
Ali J, Khan AU, Shah FA, Ali H, Islam SU, Kim YS, Khan S. Mucoprotective effects of Saikosaponin-A in 5-fluorouracil-induced intestinal mucositis in mice model. Life Sci. 2019;239:116888.
Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–27.
Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–35.
Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med. 1995;332:27–37.
Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med. 2010;181:1294–309.
Francois S, El Benna J, Dang PM, Pedruzzi E, Gougerot-Pocidalo MA, Elbim C. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol. 2005;174:3633–42.
Bonnans C, Levy BD. Lipid mediators as agonists for the resolution of acute lung inflammation and injury. Am J Respir Cell Mol Biol. 2007;36:201–5.
Fisher IM, Seropian D, Kraskauskas JN, Thakkar NF, Voelkel AA, Fowler RN 3rd. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit Care Med. 2011;39:1454–60.
Lei J, Wei Y, Song P, Li Y, Zhang T, Feng Q, Xu G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur J Pharmacol. 2018;818:110–4.
Li L, Dong L, Zhao D, Gao F, Yan J. Classical dendritic cells regulate acute lung inflammation and injury in mice with lipopolysaccharideinduced acute respiratory distress syndrome. Int J Mol Med. 2019;44:617–29.
Liu J, Huang X, Hu S, He H, Meng Z. Dexmedetomidine attenuates lipopolysaccharide induced acute lung injury in rats by inhibition of caveolin-1 downstream signaling. Biomed Pharmacother. 2019;118:109314.
Maher J, Yamamoto M. The rise of antioxidant signaling–the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010;244:4–15.
Funding
This study is funded by “The Training Program of Leading Talents of Pudong Health System, Shanghai (Grant/Award Number: PWRl2017-01),” “The Training Program of the Key Department of Pudong Health System, Shanghai (Grant/Award Number: PWZzk2017-18),” and “Young Medical Talents Training Program of Pudong Health Bureau of Shanghai (Grant/Award Number: PWRq2020-46).”
Author information
Authors and Affiliations
Contributions
Xiaorong Yang made substantial contributions to the conceptions of the study; Chengshi Zhang and Yunfeng Zhao contributed to the investigations and data curation; Xiaorong Yang drafted the manuscript. All the authors have read and approved the submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, C., Zhao, Y. & Yang, X. Azilsartan attenuates lipopolysaccharide-induced acute lung injury via the Nrf2/HO-1 signaling pathway. Immunol Res 70, 97–105 (2022). https://doi.org/10.1007/s12026-021-09240-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-021-09240-1