Abstract
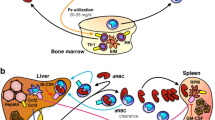
Terminal erythroid differentiation occurs in the bone marrow, within specialized niches termed erythroblastic islands. These functional units consist of a macrophage surrounded by differentiating erythroblasts and have been described more than five decades ago, but their function in the pathophysiology of erythropoiesis has remained unclear until recently. Here we propose that the central macrophage in the erythroblastic island contributes to the pathophysiology of anemia of inflammation. After introducing erythropoiesis and the interactions between the erythroblasts and the central macrophage within the erythroblastic islands, we will discuss the immunophenotypic characterization of this specific subpopulation of macrophages. We will then integrate these concepts into the currently known pathophysiological drivers of anemia of inflammation and address the role of the central macrophage in this disorder. Finally, as a means of furthering our understanding of the various concepts, we will discuss the differences between murine and rat models with regard to developmental and stress erythropoiesis in an attempt to define a model system representative of human pathophysiology.




Similar content being viewed by others
References
Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23.
Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Rev. 2002;16:87–96.
Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC, Dc W. Prevalence of anemia in persons 65 years and older in the United States : evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8.
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Zhu J, Emerson SG. Hematopoietic cytokines, transcription factors and lineage commitment. Oncogene. 2002;21:3295–313.
Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–44.
Siatecka M, Bieker JJ. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood. 2011;118:2044–54.
Olopade OI, Thangavelu M, Larson RA, Mick R, Kowal-Vern A, Schumacher HR, et al. Clinical, morphologic, and cytogenetic characteristics of 26 patients with acute erythroblastic leukemia. Blood. 1992;80:2873–82.
Frontelo P, Manwani D, Galdass M, Karsunky H, Lohmann F, Gallagher PG, et al. Novel role for EKLF in megakaryocyte lineage commitment. Blood. 2007;110:3871–80.
Hattangadi SM, Wong P, Zhang L, Flygare J, Lodish HF. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood. 2011;118:6258–68.
Tallack MR, Whitington T, Yuen WS, Kassouf MT, Hughes JR, Taylor S, et al. A global role for KLF1 in erythropoiesis revealed by ChIP-seq in primary erythroid cells. Genome Res. 2010;20:1052–63.
Koury ST, Koury MJ, Bondurant MC. Cytoskeletal distribution and function during the maturation and enucleation of mammalian erythroblasts. J Cell Biol. 1989;109:3005–13.
Skutelsky E, Danon D. An electron microscopic study of nuclear elimination from the late erythroblast. J Cell Biol. 1967;33:625–35.
McGrath KE, Kingsley PD, Koniski AD, Porter RL, Bushnell TP, Palis J. Enucleation of primitive erythroid cells generates a transient population of “pyrenocytes” in the mammalian fetus. Blood. 2008;111:2409–17.
Yoshida H, Kawane K, Koike M, Mori Y, Uchiyama Y, Nagata S. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. 2005;437:754–8.
Tavassoli M, Crosby WH. Fate of the nucleus of the marrow erythroblast. Science (80-). 1973;179:912–3.
Van Leyen K, Duvoisin RM, Engelhardt H, Wiedmann M. A function for lipoxygenase in programmed organelle degradation. Nature. 1998;395:392–5.
Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–78.
Ney PA. Normal and disordered reticulocyte maturation. Curr Opin Hematol. 2011;18:152–7.
Liu J, Guo X, Mohandas N, Chasis JA, An X. Membrane remodeling during reticulocyte maturation. Blood. 2010;115:2021–20277.
Blanc L, Vidal M. Reticulocyte membrane remodeling: contribution of the exosome pathway. Curr Opin Hematol. 2010;17:177–83.
Lacombe C, Da Silva JL, Bruneval P, Fournier JG, Wendling F, Casadevall N, et al. Peritubular cells are the site of erythropoietin synthesis in the murine hypoxic kidney. J Clin Invest. 1988;81:620–3.
Semenza GL, Koury ST, Nejfelt MK, Gearhart JD, Antonarakis SE. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc Natl Acad Sci USA. 1991;88:8725–9.
Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, et al. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116:3039–48.
Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2a is required for normal hematopoiesis in mice. Blood. 2003;102:1634–40.
Gregory CJ. Erythropoietin sensitivity as a differentiation marker in the hemopoietic system: studies of three erythropoietic colony responses in culture. J Cell Physiol. 1976;89:289–301.
Koury MJ, Bondurant MC. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248:378–81.
Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–55.
Rubiolo C, Piazzolla D, Meissl K, Beug H, Huber JC, Kolbus A, et al. A balance between Raf-1 and Fas expression sets the pace of erythroid differentiation. Blood. 2006;108:152–9.
Bessis M. Erythroblastic island, functional unity of bone marrow. Rev Hematol. 1958;13:8–11.
Mohandas N, Prenant M. Three-dimensional model of bone marrow. Blood. 1978;51:633–43.
Dzierzak E, Philipsen S. Erythropoiesis : development and Differentiation. Cold Spring Harb Perspect Med. 2013;3:1–16.
Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–8.
Hanspal M, Smockova Y, Uong Q. Molecular identification and functional characterization of a novel protein that mediates the attachment of erythroblasts to macrophages. Blood. 1998;92:2940–50.
Rhodes MM, Kopsombut P, Bondurant MC, Price JO, Koury MJ. Adherence to macrophages in erythroblastic islands enhances erythroblast proliferation and increases erythrocyte production by a different mechanism than erythropoietin. Blood. 2008;111:1700–8.
Hanspal M, Hanspal JS. The association of erythroblasts with macrophages promotes erythroid proliferation and maturation: a 30-kD heparin-binding protein is involved in this contact. Blood. 1994;84:3494–504.
Soni S, Bala S, Gwynn B, Sahr KE, Peters LL, Hanspal M. Absence of erythroblast macrophage protein (Emp) leads to failure of erythroblast nuclear extrusion. J Biol Chem. 2006;281:20181–9.
Sadahira Y, Yoshino T, Monobe Y. Very late activation antigen 4-vascular cell adhesion molecule 1 interaction is involved in the formation of erythroblastic islands. J Exp Med. 1995;181:411–5.
Lee G, Lo A, Short SA, Mankelow TJ, Spring F, Parsons SF, et al. Targeted gene deletion demonstrates that the cell adhesion molecule ICAM-4 is critical for erythroblastic island formation. Blood. 2006;108:2064–71.
De Jong JP. Voerman JS, van der, Sluijs-Gelling AJ, Willemsen R, Ploemacher RE. A monoclonal antibody (ER-HR3) against murine macrophages. I. Ontogeny, distribution and enzyme histochemical characterization of ER-HR3-positive cells. Cell Tissue Res. 1994;275:567–76.
Morris L, Crocker PR, Fraser I, Hill M, Gordon S. Expression of a divalent cation-dependent erythroblast adhesion receptor by stromal macrophages from murine bone marrow. J Cell Sci. 1991;99(Pt 1):141–7.
Fabriek BO, Polfliet MMJ, Vloet RPM, Van Der Schors RC, Ligtenberg AJM, Weaver LK, et al. The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood. 2007;109:5223–9.
Skutelsky E, Danon D. On the expulsion of the erythroid nucleus and its phagocytosis. Anat Rec. 1972;173:123–6.
Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y, et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. 2001;292:1546–9.
Lee JCM, Gimm JA, Lo AJ, Koury MJ, Krauss SW, Mohandas N, et al. Mechanism of protein sorting during erythroblast enucleation: role of cytoskeletal connectivity. Blood. 2004;103:1912–9.
Toda S, Segawa K, Nagata S. MerTK-mediated engulfment of pyrenocytes by central macrophages in erythroblastic islands. Blood. 2014;123:3963–71.
Tallack MR, Magor GW, Dartigues B, Sun L, Huang S, Fittock JM, et al. Novel roles for KLF1 in erythropoiesis revealed by mRNA-seq. Genome Res. 2012;22:2385–98.
Porcu S, Manchinu MF, Marongiu MF, Sogos V, Poddie D, Asunis I, et al. Klf1 affects DNase II-alpha expression in the central macrophage of a fetal liver erythroblastic island: a non-cell-autonomous role in definitive erythropoiesis. Mol Cell Biol. 2011;31:4144–54.
Sui Z, Nowak RB, Bacconi A, Kim NE, Liu H, Li J, et al. Tropomodulin3-null mice are embryonic lethal with anemia due to impaired erythroid terminal differentiation in the fetal liver. Blood. 2014;123:758–67.
Lee SH. Isolation and immunocytochemical characterization of human bone marrow stromal macrophages in hemopoietic clusters. J Exp Med [Internet]. 1988;168:1193–8. http://www.jem.org/cgi/doi/10.1084/jem.168.3.1193.
McKnight AJ, Macfarlane AJ, Dri P, Turley L, Willis AC, Gordon S. Molecular cloning of F4/80, a murine macrophage-restricted cell surface glycoprotein with homology to the G-protein-linked transmembrane 7 hormone receptor family. J Biol Chem. 1996;271:486–9.
Sadahira Y, Yasuda T, Kimoto T. Regulation of Forssman antigen expression during maturation of mouse stromal macrophages in haematopoietic foci. Immunology. 1991;73:498–504.
Jacobsen RN, Perkins AC, Levesque J-P. Macrophages and regulation of erythropoiesis. Curr Opin Hematol [Internet]. 2015;22:1. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00062752-900000000-99569.
Hartnell A, Steel J, Turley H, Jones M, Jackson DG, Crocker PR. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001;97:288–96.
Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med [Internet]. Nature Publishing Group; 2013;19:429–36. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3983996&tool=pmcentrez&rendertype=abstract.
Means RT. Recent developments in the anemia of chronic disease. Curr Hematol Rep. 2003;2:116–21.
Koury MJ. Abnormal erythropoiesis and the pathophysiology of chronic anemia. Blood Rev [Internet]. Elsevier B.V.; 2014;28:49–66. http://dx.doi.org/10.1016/j.blre.2014.01.002.
Baer AN, Dessypris EN, Goldwasser E, Krantz SB. Blunted erythropoietin response to anaemia in rheumatoid arthritis. Br J Haematol. 1987;66:559–64.
Hochberg MC, Arnold CM, Hogans BB, Spivak JL. Serum immunoreactive erythropoietin in rheumatoid arthritis: impaired response to anemia. Arthritis Rheum. 1988;31:1318–21.
Ganz T. Hepcidin and iron regulation. Blood. 2014;117:4425–33.
Miller CB, Jones RJ, Piantadosi S, Abeloff MD, Spivak JL. Decreased erythropoietin response in patients with the anemia of cancer. N Engl J Med. 1990;322:1689–92.
Macdougall IC, Cooper AC. Erythropoietin resistance: the role of inflammation and pro-inflammatory cytokines. Nephrol Dial Transpl. 2002;17(Suppl 1):39–43.
Bárány P. Inflammation, serum C-reactive protein, and erythropoietin resistance. Nephrol Dial Transpl. 2001;16:224–7.
Ganz T. Anemia of Chronic Disease. In: Lichtman M, Kipps T, Seligsohn U, Kaushansky K, Prchal J, editors. Williams Hematol. 8th ed. New York, Ny: McGraw-Hill; 2010.
Imagawa S, Nakano Y, Obara N, Suzuki N, Doi T, Kodama T, et al. A GATA-specific inhibitor (K-7174) rescues anemia induced by IL-1beta, TNF-alpha, or L-NMMA. FASEB J. 2003;17:1742–4.
Means RT, Krantz SB. Inhibition of human erythroid colony-forming units by gamma interferon can be corrected by recombinant human erythropoietin. Blood. 1991;78:2564–7.
Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. 1998;18:555–9.
Taniguchi S, Dai CH, Price JO, Krantz SB. Interferon gamma downregulates stem cell factor and erythropoietin receptors but not insulin-like growth factor-I receptors in human erythroid colony-forming cells. Blood. 1997;90:2244–52.
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science (80-). 2004;306:2090–3.
Pietrangelo A. Physiology of iron transport and the hemochromatosis gene. Am J Physiol Gastrointest Liver Physiol. 2002;282:G403–14.
Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44.
Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–5.
Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet [Internet]. Nature Publishing Group; 2014;46:678–84. http://www.ncbi.nlm.nih.gov/pubmed/24880340.
Kautz L, Jung G, Du X, Gabayan V, Chapman J, Nasoff M, et al. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of -thalassemia. Blood [Internet]. 2015. http://www.bloodjournal.org/cgi/doi/10.1182/blood-2015-07-658419.
Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101:4148–54.
Wang CQ, Udupa KB, Lipschitz DA. Interferon-gamma exerts its negative regulatory effect primarily the earliest stages of murine erythroid progenitor cell development. J Cell Physiol. 1995;162:134–8.
Libregts SF, Gutiérrez L, De Bruin AM, Wensveen FM, Papadopoulos P, Van Ijcken W, et al. Chronic IFN-γ production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118:2578–88.
Dai CH, Price JO, Brunner T, Krantz SB. Fas ligand is present in human erythroid colony-forming cells and interacts with Fas induced by interferon gamma to produce erythroid cell apoptosis. Blood. 1998;91:1235–42.
Felli N, Pedini F, Zeuner A, Petrucci E, Testa U, Conticello C, et al. Multiple members of the TNF superfamily contribute to IFN-gamma-mediated inhibition of erythropoiesis. J Immunol. 2005;175:1464–72.
Moldawer LL, Marano MA, Wei H, Fong Y, Silen ML, Kuo G, et al. Cachectin/tumor necrosis factor-alpha alters red blood cell kinetics and induces anemia in vivo. FASEB J. 1989;3:1637–43.
Jongen-Lavrencic M, Peeters HR, Wognum A, Vreugdenhil G, Breedveld FC, Swaak AJ. Elevated levels of inflammatory cytokines in bone marrow of patients with rheumatoid arthritis and anemia of chronic disease. J Rheumatol. 1997;24:1504–9.
Katevas P, Andonopoulos AP, Kourakli-Symeonidis A, Manopoulou E, Lafi T, Makri M, et al. Peripheral blood mononuclear cells from patients with rheumatoid arthritis suppress erythropoiesis in vitro via the production of tumor necrosis factor alpha. Eur J Haematol. 1994;53:26–30.
Voulgari PV, Kolios G, Papadopoulos GK, Katsaraki A, Seferiadis K, Drosos AA. Role of cytokines in the pathogenesis of anemia of chronic disease in rheumatoid arthritis. Clin Immunol. 1999;92:153–60.
Vreugdenhil G, Löwenberg B, Van Eijk HG, Swaak AJ. Tumor necrosis factor alpha is associated with disease activity and the degree of anemia in patients with rheumatoid arthritis. Eur J Clin Invest. 1992;22:488–93.
Papadaki HA, Kritikos HD, Valatas V, Boumpas DT, Eliopoulos GD. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood. 2002;100:474–82.
Heuser M, Ganser A. Recombinant human erythropoietin in the treatment of nonrenal anemia. Ann Hematol. 2006;85:69–78.
Ramos P, Casu C, Gardenghi S, Breda L, Crielaard BJ, Guy E, et al. Macrophages support pathological erythropoiesis in polycythemia vera and β-thalassemia. Nat Med [Internet]. Nature Publishing Group; 2013;19:437–45. http://www.ncbi.nlm.nih.gov/pubmed/23502961.
Angelillo-Scherrer A, Burnier L, Lambrechts D, Fish RJ, Tjwa M, Plaisance S, et al. Role of Gas6 in erythropoiesis and anemia in mice. J Clin Invest. 2008;118:583–96.
Sawada K, Krantz SB, Dessypris EN, Koury ST, Sawyer ST. Human colony-forming units-erythroid do not require accessory cells, but do require direct interaction with insulin-like growth factor I and/or insulin for erythroid development. J Clin Invest. 1989;83:1701–9.
Means RT. Hepcidin and cytokines in anaemia. Hematology. 2004;9:357–62.
De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, et al. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature. 1999;401:489–93.
Dai C, Chung IJ, Jiang S, Price JO, Krantz SB. Reduction of cell cycle progression in human erythroid progenitor cells treated with tumour necrosis factor alpha occurs with reduced CDK6 and is partially reversed by CDK6 transduction. Br J Haematol. 2003;121:919–27.
Flores-Figueroa E, Gutiérrez-Espíndola G, Montesinos JJ, Arana-Trejo RM, Mayani H. In vitro characterization of hematopoietic microenvironment cells from patients with myelodysplastic syndrome. Leuk Res. 2002;26:677–86.
Maddala R, Reddy VN, Epstein DL, Rao V. Growth factor induced activation of Rho and Rac GTPases and actin cytoskeletal reorganization in human lens epithelial cells. Mol Vis. 2003;9:329–36.
Zamai L, Secchiero P, Pierpaoli S, Bassini A, Papa S, Alnemri ES, et al. TNF-related apoptosis-inducing ligand (TRAIL) as a negative regulator of normal human erythropoiesis. Blood. 2000;95:3716–24.
Secchiero P, Melloni E, Heikinheimo M, Mannisto S, Di Pietro R, Iacone A, et al. TRAIL regulates normal erythroid maturation through an ERK-dependent pathway. Blood. 2004;103:517–22.
Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95.
Recalcati S, Locati M, Marini A, Santambrogio P, Zaninotto F, De Pizzol M, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol. 2010;40:824–35.
Gammella E, Buratti P, Cairo G, Recalcati S. Macrophages: central regulators of iron balance. Metallomics [Internet]. 2014;6:1336–42. http://pubs.rsc.org/en/Content/ArticleLanding/2014/MT/C4MT00104D#!divAbstract.
Vallelian F, Schaer CA, Kaempfer T, Gehrig P, Duerst E, Schoedon G, et al. Glucocorticoid treatment skews human monocyte differentiation into a hemoglobin-clearance phenotype with enhanced heme-iron recycling and antioxidant capacity. Blood. 2010;116:5347–56.
Iavarone A, King ER, Dai X-M, Leone G, Stanley ER, Lasorella A. Retinoblastoma promotes definitive erythropoiesis by repressing Id2 in fetal liver macrophages. Nature. 2004;432:1040–5.
Clarke AR, Maandag ER, van Roon M, van der Lugt NM, van der Valk M, Hooper ML, et al. Requirement for a functional Rb-1 gene in murine development. Nature. 1992;359:328–30.
Clark AJ, Doyle KM, Humbert PO. Cell-intrinsic requirement for pRb in erythropoiesis. Blood. 2004;104:1324–6.
Sankaran VG, Orkin SH, Walkley CR. Rb intrinsically promotes erythropoiesis by coupling cell cycle exit with mitochondrial biogenesis. Genes Dev. 2008;22:463–75.
Kataoka K, Noda M, Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol Cell Biol. 1994;14:700–12.
Yoshida T, Ohkumo T, Ishibashi S, Yasuda K. The 5′-AT-rich half-site of Maf recognition element: a functional target for bZIP transcription factor Maf. Nucleic Acids Res. 2005;33:3465–78.
Kusakabe M, Hasegawa K, Hamada M, Nakamura M, Ohsumi T, Suzuki H, et al. c-Maf plays a crucial role for the definitive erythropoiesis that accompanies erythroblastic island formation in the fetal liver. Blood. 2011;118:1374–85.
Yien Y, Bieker JJ. EKLF/KLF1: a tissue-restricted integrator of transcriptional control, chromatin remodeling, and lineage determination. Mol Cell Biol. 2013;33:4–13.
Xue L, Galdass M, Gnanapragasam MN, Manwani D, Bieker JJ. Extrinsic and intrinsic control by EKLF (KLF1) within a specialized erythroid niche. Development [Internet]. 2014;141:2245–54. http://www.ncbi.nlm.nih.gov/pubmed/24866116.
Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–16.
Piomelli S, Seaman C. Mechanism of red blood cell aging: relationship of cell density and cell age. Am J Hematol. 1993;42:46–52.
Lutz HU. Innate immune and non-immune mediators of erythrocyte clearance. Cell Mol Biol (Noisy-le-grand). 2004;50:107–16.
Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, Vandivier RW, Harry BL, Riches DWH, et al. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113:2047–55.
Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–4.
Melhorn MI, Brodsky AS, Estanislau J, Khoory JA, Illigens B, Hamachi I, et al. CR1-mediated ATP release by Human red blood cells promotes CR1 clustering and modulates the immune transfer process. J Biol Chem. 2013;288:31139–53.
White C, Yuan X, Schmidt PJ, Bresciani E, Samuel TK, Campagna D, et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab [Internet]. 2013;17:261–70. http://linkinghub.elsevier.com/retrieve/pii/S1550413113000132.
Soe-Lin S, Sheftel AD, Wasyluk B, Ponka P. Nramp1 equips macrophages for efficient iron recycling. Exp Hematol. 2008;36:929–37.
Bessis MC, Breton-Gorius J. Iron metabolism in the bone marrow as seen by electron microscopy: a critical review. Blood. 1962;19:635–63.
Leimberg MJ, Prus E, Konijn AM, Fibach E. Macrophages function as a ferritin iron source for cultured human erythroid precursors. J Cell Biochem. 2008;103:1211–8.
Baron MH, Isern J, Fraser ST. The embryonic origins of erythropoiesis in mammals. Blood. 2012;119:4828–37.
Bradley A. Mining the mouse genome. Nature. 2002;420:512–4.
Mikkola HKA, Orkin SH. Gene targeting and transgenic strategies for the analysis of hematopoietic development in the mouse. Methods Mol Med. 2005;105:3–22.
Paigen K. One hundred years of mouse genetics: an intellectual history. II. The molecular revolution (1981–2002). Genetics. 2003;163:1227–35.
Palis J. Ontogeny of erythropoiesis. Curr Opin Hematol. 2008;15:155–61.
Tavian M, Péault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49:243–50.
Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, De Mesy-Bentley KKL, et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109:1433–41.
Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–84.
Isern J, He Z, Fraser ST, Nowotschin S, Ferrer-Vaquer A, Moore R, et al. Single-lineage transcriptome analysis reveals key regulatory pathways in primitive erythroid progenitors in the mouse embryo. Blood. 2011;117:4924–34.
Fraser ST, Isern J, Baron MH. Maturation and enucleation of primitive erythroblasts during mouse embryogenesis is accompanied by changes in cell-surface antigen expression. Blood. 2007;109:343–52.
Kingsley PD, Malik J, Fantauzzo KA, Palis J. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood. 2004;104:19–25.
Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–9.
Cumano A, Godin I. The ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–85.
McGrath KE, Frame JM, Fromm GJ, Koniski AD, Kingsley PD, Little J, et al. A transient definitive erythroid lineage with unique regulation of the Beta-globin locus in the mammalian embryo. Blood. 2011;117:4600–8.
Allen TD, Dexter TM. Ultrastructural aspects of erythropoietic differentiation in long-term bone marrow culture. Differentiation. 1982;21:86–94.
Brodsky I, Dennis LH, Kahn SB, Brady LW. Normal mouse erythropoiesis. I. The role of the spleen in mouse erythropoiesis. Cancer Res. 1966;26:198–201.
Vacek A, Bartonickova A, Tkadlecek L. Age dependence of the number of the stem cells in haemopoietic tissues of rats. Cell Tissue Kinet. 1976;9:1–8.
Garcia JF. Changes in blood, plasma and red cell volume in the male rat, as a function of age. Am J Physiol. 1957;190:19–24.
Seifert MF, Marks SC. The regulation of hemopoiesis in the spleen. Experientia. 1985;41:192–9.
Socolovsky M. Molecular insights into stress erythropoiesis. Curr Opin Hematol. 2007;14:215–24.
Lenox LE, Perry JM, Paulson RF. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood. 2005;105:2741–8.
Paulson RF, Shi L, Wu D-C. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol. 2011;18:139–45.
Maekawa S, Iemura H, Kato T. Enhanced erythropoiesis in mice exposed to low environmental temperature. J Exp Biol [Internet]. 2013;216:901–8. http://www.ncbi.nlm.nih.gov/pubmed/23155089.
Bauer A, Tronche F, Wessely O, Kellendonk C, Reichardt HM, Steinlein P, et al. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999;13:2996–3002.
Broudy VC, Lin NL, Priestley GV, Nocka K, Wolf NS. Interaction of stem cell factor and its receptor c-kit mediates lodgment and acute expansion of hematopoietic cells in the murine spleen. Blood. 1996;88:75–81.
Millot S, Andrieu V, Letteron P, Lyoumi S, Hurtado-Nedelec M, Karim Z, et al. Erythropoietin stimulates spleen BMP4-dependent stress erythropoiesis and partially corrects anemia in a Mouse model of generalized inflammation. Blood. 2010;116:6072–81.
Perry JM, Harandi OF, Paulson RF. BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood. 2007;109:4494–502.
Lenox LE, Shi L, Hegde S, Paulson RF. Extramedullary erythropoiesis in the adult liver requires BMP-4/Smad5-dependent signaling. Exp Hematol. 2009;37:549–58.
Ploemacher RE, van Soest PL. Morphological investigation on phenylhydrazine-induced erythropoiesis in the adult mouse liver. Cell Tissue Res. 1977;178:435–61.
Ploemacher RE, van Soest PL, Vos O. Kinetics of erythropoiesis in the liver induced in adult mice by phenylhydrazine. Scand J Haematol. 1977;19:424–34.
Nagai Hirofumi, Chatani Fumio, Sasaki Satoshi, Miyajima H. Negligible contribution of splenic erythropoiesis to recovery from anemia in rats. J Toxicol Pathol. 1993;6:241–9.
Migliaccio AR, Whitsett C, Papayannopoulou T, Sadelain M. The potential of stem cells as an in vitro source of red blood cells for transfusion. Cell Stem Cell [Internet]. Elsevier Inc.; 2012;10:115–9. http://dx.doi.org/10.1016/j.stem.2012.01.001.
Douay L, Andreu G. Ex vivo production of human red blood cells from hematopoietic stem cells: What is the future in transfusion? Transfus Med Rev. 2007;21:91–100.
Giarratana M-C, Kobari L, Lapillonne H, Chalmers D, Kiger L, Cynober T, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74.
Acknowledgments
We apologize for being unable to cite numerous studies due to space restrictions. This work was supported by NIH (DK26263 to NM) and the Pediatric Cancer Foundation (LB). BMD is a recipient of an American Society of Hematology Physician-Scientist Career Development Award. LB is the recipient of an Allied World St. Baldrick’s Scholar Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hom, J., Dulmovits, B.M., Mohandas, N. et al. The erythroblastic island as an emerging paradigm in the anemia of inflammation. Immunol Res 63, 75–89 (2015). https://doi.org/10.1007/s12026-015-8697-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-015-8697-2