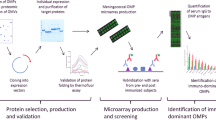
Abstract
Outer-membrane vesicles (OMVs) have inherent adjuvant properties, and many vaccines use OMV as vaccine components. Utilizing the adjuvant properties of OMV could lead to the formulation of vaccines that are less expensive and potentially more immunogenic than covalently conjugated polysaccharide vaccines. We evaluated the adjuvant effect in Balb/c mice of combinations of OMV from Neisseria meningitidis serogroup A and W135 as compared to that of the non-covalently conjugated capsular polysaccharide A. Both antigens were adsorbed onto aluminum hydroxide. The mice were given a booster dose of plain polysaccharide A to stimulate an immunologic memory response. Subclasses determination and cytokine assays demonstrated the capacity of OMV to induce a IgG2a/IgG2b isotype profile and IFN-γ production, suggesting the induction of a Th1 pattern immune response. Lymphoproliferative responses to OMVs were high, with affinity maturation of antibodies observed. Bactericidal titers after the booster dose were also observed. Memory B cells and long-term memory T cells were also detected. The results of this study indicate that combined meningococcal serogroup A and W135 OMV can activate cell-mediated immunity and induce a long-term memory response.






Similar content being viewed by others
References
Weintraub A. Immunology of bacterial polysaccharide antigens. Carb Res. 2003;338:2539–47.
Wing J, Smart L, Borrow R, Findlow J, Findlow H, Lees A, Read RC, et al. Correlation of group C meningococcal conjugate vaccine response with B- and T-lymphocyte activity. PLoS ONE. 2012;7:e31160.
Ambrosino DM, Sood SK, Lee MC, Chen D, Collard HR, Bolon DL, Johnson C, et al. IgG1, IgG2 and IgM responses to two Haemophilus influenzae type b conjugate vaccines in young infants. Pediatr Infect Dis J. 1992;11:855–9.
Borrow R, Southern J, Andrews N, Peake N, Rahim R, Acuna M, Martin S, et al. Comparison of antibody kinetics following meningococcal serogroup C conjugate vaccine between healthy adults previously vaccinated with meningococcal A/C polysaccharide vaccine and vaccine-naïve controls. Vaccine. 2001;19:3043–50.
Goldblatt D, Vaz AR, Miller E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J Infect Dis. 1998;177:1112–5.
Gourley TS, Wherry EJ, Masopust D, Ahmed R. Generation and maintenance of immunological memory. Semin Immunol. 2004;16:323–33.
Holst J, Martin D, Arnold R, Campa C, Oster P, O’Hallahan J, Rosenqvist E. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27S:B3–12.
Sierra G, Campa C, Varcárcel M. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Annals. 1991;14:195–210.
Pérez O, Mastroeni P, Rodríguez T et al. Proteoliposomes and derivatives thereof as cytotoxic response-inducing adjuvants and resulting formulations EP1716866 A1; 2003.
Pérez O, Bracho G, Lastre M, Sierra G, Campa C, Mora N, del Campo J et al. Método de obtención de estructuras cocleares, Composiciones vacunales y adyuvantes basados en estructuras cocleares y sus intermediarios. Patent application Cu 2002–0292.
Rodríguez T, Pérez O, Ménager N, Ugrinovic S, Bracho G, Mastroeni P. Interactions of proteoliposomes from serogroup B Neisseria meningitidis with bone marrow-derived dendritic cells and macrophages: adjuvant effects and antigen delivery. Vaccine. 2005;23:1312–21.
Pérez O, Lastre M, Lapinet J, Bracho G, Díaz M, Zayas C, et al. Immune response induction and new effector mechanisms possibly involved in protection conferred by the Cuban Anti-Meningococcal BC Vaccine. Infect Immun. 2001;2001(6):4502–8.
Pérez O, Lastre M, Lapinet J, Pérez A, Díaz M, Zayas C, et al. Long-lasting cellular immune response in babies, children, and pre-teenagers vaccinated with a proteoliposome based anti-meningococcal BC vaccine. Inmunología. 2001;20:177–83.
Camaraza M, Ochoa R, Arnet A, Sotolongo F, Martínez I, Cuevas I, Hernández D. Inmunogenicidad inducida por la vacuna antimeningocócica VA-MENGOC-BC® contra la cepa de N. meningitidis ATCC C11 en adolescentes después de 12 años de vacunados. Rev Cuba Med Trop. 2004;56:26–30.
Pérez O, Romeu B, Lastre M, Gonzalez E, Balboa J, Zayas C. et al. Adjuvants to polysaccharides vaccines. Patent number 2011 CU-P-2011-2002.
Norheim G, Tunheim G, Næss LM, Kristiansen P, Caugant D, Rosenqvist E. An outer membrane vesicle vaccine for prevention of serogroup A and W-135 meningococcal disease in the African meningitis belt Scand. J Immunol. 2012;76:99–107.
Campa C, Sierra V, Gutiérrez M, Biset G, García L, Puentes G, Sampedro M. et al. Method of producing Neisseria meningitidis B vaccine, and vaccine produced by method. United States Patent 1997. Patent Number: 5,597,572.
Norheim G, Aase A, Caugant D, Høiby E, Fritzsønn E, Tangen T, Kristiansen P, et al. Development and characterisation of outer membrane vesicle vaccines against serogroup A Neisseria meningitidis. Vaccine. 2005;23:3762–74.
Romeu B, González E, Zayas C, Del Campo J, Acevedo R, Cuello M, Valdes Y, et al. AFCo1 as nasal adjuvant of capsular polysaccharide from Neisseria meningitidis serogroup C induces systemic and mucosal immune responses. Scand J Infect Dis. 2011;43:809–13.
Anttila M, Eskola J, Ahman H, Kayht H. Avidity of IgG for Streptococcus pneumoniae Type 6B and 23F Polysaccharides in Infants Primed with Pneumococcal Conjugates and Boosted with Polysaccharide or Conjugate Vaccines J. Infect Dis. 1998;177:1614–21.
Norheim G, Arne Høiby E, Caugant DA, Namork E, Tangen T, Fritzsønn E, Rosenqvist E. Immunogenicity and bactericidal activity in mice of an outer membrane protein vesicle vaccine against Neisseria meningitidis serogroup A disease. Vaccine. 2004;22:2171–80.
Mata E, Igartua M, Patarroyo ME, Pedraz JL, Hernández R. Enhancing immunogenicity to PLGA microparticulate systems by incorporation of alginate and RGD-modified alginate Eur. J Pharm Sci. 2011;44:32–40.
Carcaboso AM, Hernandez RM, Igartua M, Rosas JE, Patarroyo ME, Pedraz JL. Potent, long lasting systemic antibody levels and mixed Th1/Th2 immune response after nasal immunization with malaria antigen loaded PLGA microparticles. Vaccine. 2004;22:1423–32.
Hennekena M, Burdinb N, Thoroddsenc E, Sigurdardottir S, Trannoyb E, Jonsdottir I. Meningococcal serogroup C polysaccharide specific memory B cells, directly enumerated by labeled polysaccharide, are not affected by age at vaccination. Vaccine. 2010;28:2097–103.
Keating SM, Bejon P, Berthoud T, Vuola J, Todryk S, Webster D, Dunachie S, et al. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J Immunol. 2005;175:5675–80.
Trotter C, McVernon J, Ramsay M, Whitney C, Mulholland E, Goldblatt D, Hombach J, et al. Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine. 2008;26:4434–45.
Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–20.
Granoff D, Pollard A. Reconsideration of the use of meningococcal polysaccharide vaccine. Ped Infect Dis J. 2007;26:716–22.
Pérez O, Bracho G, Lastre M, Mora N, del Campo J, Gil D, Zayas C, et al. Novel adjuvant based on a proteoliposome-derived cochleate structure containing native lipopolysaccharide as a pathogen associated molecular pattern. Immunol Cell Biol. 2004;82:603–10.
Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines. 2011;10:307–22.
Joseph H, Ryall R, Bybel M, Papa T, MacLennan J, Buttery J, Borrow R. Immunogenicity and immunological priming of the serogroup A portion of a bivalent meningococcal A/C conjugate vaccine in 2-year-old children. J Infect Dis. 2003;187:1142–6.
Reingold AL, Broome CV, Hightower A, Ajello G, Bolan G, Adamsbaum C, Jones E, et al. Age-specific differences in duration of clinical protection after vaccination with meningococcal polysaccharide A vaccine. Lancet. 1985;326:114–8.
Ismail AA, Harris SL, Granoff DM. Serum group A anticapsular antibodies in a Sudanese population immunized with meningococcal polysaccharide vaccine during a group A epidemic. Pediatr Infect Dis J. 2004;23:748–55.
Lambert PH, Liu M, Siegrist CA. Can successful vaccines teach us how to induce efficient protective immune responses? Nat Med. 2005;11:S54–62.
Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89.
Taillardet M, Haffar G, Mondie P, Asensio MJ, Pleau-Pison T, Burdin N, Defrance T, et al. Toll-like receptor agonists allow generation of long-lasting Antipneumococcal humoral immunity in response to a plain Polysaccharidic Vaccine. J Infect Dis. 2010;202:470–9.
Gourley T, Wherry EJ, Masopust D, Ahmed R. Generation and maintenance of immunological memory. Semin Immunol. 2004;16:323–33.
Heyzer-Williams LJ, Heyzer-Williams MG. Antigen-specific memory B cell development. Ann Rev Immunol. 2005;23:487–513.
Deauvieau F, Dussurgey S, Rossignol D, de Montfort A, Burdin N, Guy B. Memory B and T cell responses induced by serotype 4 Streptococcus pneumonia vaccines: longitudinal analysis comparing responses elicited by free polysaccharide, conjugate and carrier. Vaccine. 2010;28:576–82.
Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. 2006;203:305–10.
Clarke ET, Williams NA, Dull PM, Findlow J, Borrow R, Finn A, Heyderman RS. Polysaccharide–protein conjugate vaccination induces antibody production but not sustained B-cell memory in the human nasopharyngeal mucosa. Mucosal Immunol. 2013;6:288–96.
Saunders NB, Shoemaker DR, Brandt BL, Moran EE, Larsen T, Zollinger WD. Immunogenicity of intranasally administered meningococcal native outer membrane vesicles in mice. Infect Immun. 1999;67:113–9.
Campo JD, Zayas C, Romeu B, Acevedo R, González E, Bracho G, Cuello M, Cabrera O, Balboa J, Lastre M. Mucosal immunization using proteoliposome and cochleate structures from Neisseria meningitidis serogroup B induce mucosal and systemic responses. Methods. 2009;49:301–8.
Avci F, Kasper DL. How bacterial carbohydrates influence the adaptive immune system. Ann Rev Immunol. 2010;28:107–30.
Butler NS, Nolz JC, Harty JT. Immunologic considerations for generating memory CD8 T cells through vaccination Cell. Microbiol. 2011;13:925–33.
Avci F, Li X, Tsuji M, Kasper D. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nature Med. 2011;17:1602–9.
Acknowledgments
We would like to thank Finlay Institute for the support and sponsorship of this work. We gratefully acknowledge the help of Professor Nelson Fernández from University of Essex, UK, for reading the manuscript. We also thank Laura Reyes, Yusnaby Borrero, and Diandra Lescaille for their excellent technical assistance. We thank Dr. Daniel Cardoso, Vice president of Finlay Institute, and Dr. Reinaldo Acevedo for supporting this work. Also, we would like to thank the NFR GLOBVAC project “Development of an outer membrane vesicle vaccine for Africa, against serogroup A and W135 meningococcal disease” Project number: 192477 and Dr. Einar Rosenqvist, Dr Gunnstein Norheim, Dr. Lisbeth Naess, and Dr. Gro Turheim from Division of Infectious Disease Control, Norwegian Institute of Public Health, Oslo, Norway, for supporting the OMV from serogroup A and W135 production and characterization.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romeu, B., Lastre, M., García, L. et al. Combined meningococcal serogroup A and W135 outer-membrane vesicles activate cell-mediated immunity and long-term memory responses against non-covalent capsular polysaccharide A. Immunol Res 58, 75–85 (2014). https://doi.org/10.1007/s12026-013-8427-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-013-8427-6