Abstract
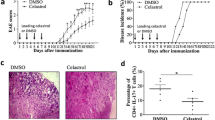
Bowman–Birk Inhibitor (BBI), a serine protease inhibitor derived from soybeans, has anti-inflammatory properties and is able to suppress the development of central nervous system (CNS) autoimmunity in animal models. Experimental autoimmune encephalomyelitis (EAE), a widely used animal model of multiple sclerosis (MS), is characterized by breakdown of the blood–brain barrier and infiltration of inflammatory cells into the CNS, resulting in pathology. In this study, we observed that BBI-treated mice showed delayed onset of EAE and reduced disease severity compared to control mice. BBI-treated mice had fewer inflammatory cells in the CNS including significantly reduced numbers of Th1 and Th17 cells. In the periphery, BBI treatment suppressed the development of encephalitogenic Th1 and Th17 responses early on [day 7 post-immunization (p.i.)], while after disease onset (day 14 p.i.) BBI-treated mice had stronger Th responses, as determined by antigen-specific proliferation and cytokine production. These results demonstrate that BBI treatment temporarily suppressed the development of encephalitogenic responses, but these responses eventually attained normal magnitude. Given that BBI-treated mice exhibited stronger encephalitogenic responses in the periphery during clinically manifesting EAE, delayed disease onset, and reduced numbers of CNS-infiltrating cells, it appears likely that BBI impedes the exit of pathogenic Th1 and Th17 cells from lymphoid organs, thereby delaying their migration into the CNS.





Similar content being viewed by others
References
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–52.
Frohman EM, Racke MK, Raine CS. Multiple sclerosis–the plaque and its pathogenesis. N Engl J Med. 2006;354:942–55.
Holmoy T. The immunology of multiple sclerosis: disease mechanisms and therapeutic targets. Minerva Med. 2008;99:119–40.
Wingerchuk DM. Current evidence and therapeutic strategies for multiple sclerosis. Semin Neurol. 2008;28:56–68.
Ransohoff RM. Natalizumab, multiple sclerosis, and primary central nervous system lymphoma: enigma, wrapped in mystery, enclosed in conundrum. Ann Neuro. 2009;66:259–61.
Furlan R, Cuomo C, Martino G. Animal models of multiple sclerosis. Methods Mol Biol. 2009;549:157–73.
Stromnes IM, Goverman JM. Passive induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1952–60.
Korn T, Mitsdoerffer M, Kuchroo VK. Immunological basis for the development of tissue inflammation and organ-specific autoimmunity in animal models of multiple sclerosis. Results Probl Cell Differ. 2009;51:43–74.
Birk Y. The Bowman-Birk inhibitor trypsin- and chymotrypsin-inhibitor from soybeans. Int J Pept Protein Res. 1985;25:113–31.
Odani S, Ikenaka T. Studies on soybean trypsin inhibitors 8 disulfide bridges in soybean Bowman-Birk proteinase inhibitor. J Biochem. 1973;74:697–715.
Marin-Manzano MC, Ruiz R, Jimenez E, Rubio LA, Clemente A. Anti-carcinogenic soyabean Bowman-Birk inhibitors survive faecal fermentation in their active form and do not affect the microbiota composition in vitro. Br J Nutr. 2009;101:967–71.
Park JH, Jeong HJ, Lumen BO. In vitro digestibility of the cancer-preventive soy peptides lunasin and BBI. J Agric Food Chem. 2007;55:10703–6.
Losso JN. The biochemical and functional food properties of the bowman-birk inhibitor. Crit Rev Food Sci Nutr. 2008;48:94–118.
Ware JH, Wan XS, Rubin H, Schechter NM, Kennedy AR. Soybean Bowman-Birk protease inhibitor is a highly effective inhibitor of human mast cell chymase. Arch Biochem Biophys. 1997;344:133–8.
Gladysheva IP, Larionova NI, Gladyshev DP, Tikhonova TV, Kazanskaia NF. The classical Bowman-Birk soy inhibitor is an effective inhibitor of human granulocyte alpha-chymotrypsin and cathepsin G. Biokhimiia. 1994;59:513–8.
Larionova NI, Gladysheva IP, Tikhonova TV, Kazanskaia NF. Inhibition of cathepsin G and elastase from human granulocytes by multiple forms of the Bowman-Birk type of soy inhibitor. Biokhimiia. 1993;58:1437–44.
Tikhonova TV, Gladysheva IP, Kazanskaia NF, Larionova NI. Inhibition of elastin hydrolysis, catalyzed by human leukocyte elastase and cathepsin G, by the Bowman-Birk type soy inhibitor. Biokhimiia. 1994;59:1739–45.
Kennedy AR. The Bowman-Birk inhibitor from soybeans as an anticarcinogenic agent. Am J Clin Nutr. 1998;68:1406S–12S.
Maki PA, Kennedy AR. Humoral and cellular immune functions are not compromised by the anticarcinogenic Bowman-Birk inhibitor. Nutr Cancer. 1992;18:165–73.
Touil T, Ciric B, Ventura E, Shindler KS, Gran B, Rostami A. Bowman-Birk inhibitor suppresses autoimmune inflammation and neuronal loss in a mouse model of multiple sclerosis. J Neurol Sci. 2008;271:191–202.
El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–67.
Li J, Ye L, Cook DR, Wang X, Liu J, Kolson DL, Persidsky Y, Ho WZ. Soybean-derived Bowman-Birk inhibitor inhibits neurotoxicity of LPS-activated macrophages. J Neuroinflammation. 2011;8:15.
Mekala DJ, Alli RS. Geiger TL.IL-10-dependent suppression of experimental allergic encephalomyelitis by Th2-differentiated, anti-TCR redirected T lymphocytes. J Immunol. 2005;174:3789–97.
Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–9.
Frenkel K, Chrzan K, Ryan CA, Wiesner R, Troll W. Chymotrypsin-specific protease inhibitors decrease H2O2 formation by activated human polymorphonuclear leukocytes. Carcinogenesis. 1987;8:1207–12.
Ware JH, Wan XS, Kennedy AR. Bowman-Birk inhibitor suppresses production of superoxide anion radicals in differentiated HL-60 cells. Nutr Cancer. 1999;33:174–7.
Ware JH, Wan XS, Newberne P, Kennedy AR. Bowman-Birk inhibitor concentrate reduces colon inflammation in mice with dextran sulfate sodium-induced ulcerative colitis. Dig Dis Sci. 1999;44:986–90.
Dia VP, Berhow MA, Gonzalez De Mejia E. Bowman-Birk inhibitor and genistein among soy compounds that synergistically inhibit nitric oxide and prostaglandin E2 pathways in lipopolysaccharide-induced macrophages. J Agric Food Chem. 2008;56:11707–17.
Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69:759–77.
Acknowledgments
This work was supported by NIH grant (R01AT005322-01) to A.R. We would like to thank Katherine Regan and Carey Myers for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, H., Ciric, B., Zhang, GX. et al. Bowman–Birk inhibitor attenuates experimental autoimmune encephalomyelitis by delaying infiltration of inflammatory cells into the CNS. Immunol Res 51, 145–152 (2011). https://doi.org/10.1007/s12026-011-8254-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-011-8254-6