Abstract
In order to evaluate immunogenicity and protective efficacy of LytA from Streptococcus pneumoniae, we subcloned the full-length lytA-encoded autolysin (LytA) from 5 major pathogenic serotype isolates in China and obtained purified rLytA. Bioinformatics analysis showed that sequences of LytA were highly conserved in all strains we used in this work, and western blot analysis demonstrated that rLytAs from heterogeneous serotypes were cross-recognized by serum of mice infected with 23F strain SH137. Mice were intranasally immunized with purified rLytA, and serum anti-rLytA IgG, IgA and secretory IgA were elicited. More importantly, rLytA intranasal-immunized mice showed a significantly higher survival rate and lower bacterial carriage in response to infection by Streptococcus pneumoniae. The fact that mice immunized with rLytA from strain SH137 also had a higher survival rate after intraperitoneal injection of other four serotype strains of living S. pneumoniae suggested that it possessed cross-protection effect. Our study revealed that intranasal immunization with rLytA may protect mice against mucosal and systemic pneumococcal infection; hence, it was an attractive vaccine candidate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus pneumoniae (S. pneumoniae) is one of the major pathogenic bacteria and main cause of acute otitis, sinusitis, meningitis and community-acquired pneumonia. All these infections cause substantial morbidity and mortality worldwide, especially in infants, the aged and immunodeficiency patients. Unfortunately, precise mechanism of S. pneumoniae pathogenesis remains unclear, and problem of multiple antibiotic resistances is becoming acute. Control of pneumococcal disease is also complicated because of suboptimal clinical efficacy of current polyvalent pneumococcal polysaccharide vaccines in high-risk groups [1]. The fact that more than 20 serotype strains of S. pneumoniae cause pneumococcal disease makes vaccine design complicated. Currently licensed vaccines available for immunoprophylactic control are the first-generation 23-valent pneumococcal capsular polysaccharide (PS) vaccine and the second-generation 7-valent PS conjugate vaccine (PCV-7). Both of them have major limitations, such as the PS vaccine is ineffective in children <2 years of age, elderly and immunocompromised individuals who actually are the most associated with infection groups [2]. A limited amount of cross-protection among serotypes had been observed with the PCV-7 vaccine, and the complexity of conjugating made them expensive to produce and difficult for wide use at present [3]. All these difficulties highlight the need for novel vaccine development.
Among the most well-characterized virulence factors of S. pneumoniae, lytA-encoded major autolysin (LytA) is responsible for cellular autolysis, through which it mediates release of toxic substances that damage host tissues then cause pneumonia, meningitis or bacteremia [4]. It is also the first example of a bacterial autolytic enzyme characterized at the molecular level [5] and has been extensively studied from the enzymatic, genetic and structural viewpoints. Some reports showed that LytA induced protective immunity [6] and as one of the potential protein vaccine candidates, it may induce T-cell-dependent and non-serotype-specific protection to a broader target population.
Human nasopharyngeal carriage of S. pneumoniae constitutes a natural bacterial reservoir and is proposed to be a prelude of virtually all pneumococcal diseases. Mucosal immunization is an effective method to block infection and colonization of S. pneumoniae and therefore reduces carriage and pneumococcal transmission; consequently, disease might be largely eliminated [7, 8]. Thus, mucosal immunity may be an ideal choice to develop vaccine; moreover, if vaccine could be administered simply to mucosal surface, immunization practice would become safer, more acceptable and more suitable for mass use [9]. In addition, mucosal vaccination should most efficiently induce immune exclusion—a term coined for non-inflammatory antibody protection at mucosal surfaces, mediated principally by immunoglobulin A (IgA) in cooperation with innate non-specific defense factors, thus referring to the ‘first line’ of microbial defense [5, 10].
Based on its capsular polysaccharide, S. pneumoniae was divided into 91 serotypes, but only about 20 serotypes cause human diseases. In China, the most pathogenic bacteria were limited to a few strains: 19F, 14, 6A, 23F and 6B [11]. We reported here a detailed analysis of genetic, protein diversity of lytA in 5 different serotype isolates from China and evaluated whether mucosal (intranasal) immunization with rLytA from one strain would induce protective immunity against local and systemic pneumococcal infection caused by all the five pathogenic strains infection in mice model.
Materials and methods
Bacterial strains, adjuvant and mice
Streptococcus pneumoniae clinical isolates SH69 (capsule type 19F), SH84 (capsule type 14), SH90 (capsule type 6A), SH137 (capsule type 23F) and SH143 (capsule type 6B) (from Public Healthy School of FuDan University) are common pathogenic serotype strains with multiple drug resistance and penicillin intermediate resistance in China [11], among which capsule type 23F (strain SH137) is one of the most common causes of invasive disease in young children and relatively non-invasive in mice, so it colonizes the upper respiratory tract in the absence of bacteremia [12]; therefore, rLytA from SH137 was chosen to infect mice. Beijing standard 31108 was used as experiment quality control, and E. coli BL21 served as host for expression vector pGEX-4T-1, which contains a GST tag (Amersham Biosciences).
CpG ODN 1,826 with sequence TCCATGACGTTCCTGACGTT from Shanghai ShengGong company was used as mucosal adjuvant for it has the strongest immunostimulator on mouse immune cells in vitro [13, 14].
Studies were carried out on inbred female BALB/c mice, 6–8 weeks old, specific pathogen free, and were obtained from Center of Animal Laboratory of Sun Yat-Sen University. All animals were maintained and used in accordance with all relevant guidelines and institutional policies.
Cloning, expression of recombinant LytA in E.coli and data analysis
Streptococcus pneumoniae were grown on blood agar base supplemented with sterile 5% defibrinated sheep blood at 37°C for about 18 h, then bacteria were collected and DNA was isolated by using DNA extraction kid from Gene com. Lid. Two oligonucleotide primers were designed according to lytA gene sequence of R6 strain: forward, 5′-GCCCCGGGTATGGAAATTAATGTGAG-3′ (with an underlined Sma I site); reverse, 5′-GCCTCGAGTTATTTTACTGTAATCAAGCC-3′ (with an underlined Xho I site). PCR was performed in a total reaction volume of 50 μl for 30 cycles consisting of a denaturation step at 94°C for 1 min, a primer annealing step at 55°C for 1 min and a primer extension step at 72°C for 2 min. Then, the LytA gene was ligated into the Sma I site and Xho I site of pGEX-4T-1 to generate recombinant plasmid pGEX/lytA for sequence confirmation. DNA sequencing was performed by ShengGong company and TaKaRa Biotechnology (Dalian) Co. Ltd independently. Nucleotide and deduced amino acid sequences of lytA from different strains were analyzed by bioinformatics tools at the World Wide Web molecular biology server (http://www.expasy.org/tools) and Biology WorkBench version 3.2 [15] (http://workbench.sdsc.edu/).
Recombinant plasmid pGEX/lytA was transformed into E. coli BL21 by heat shock method, and BL21/pGEX/lytA cells were induced for protein expression overnight at 28°C by isopropyl -d-thiogalactopyranoside (IPTG) at a final concentration of 1 mM. SDS–PAGE gel was used to identify desired protein, then expressed protein was purified first by GSTrap FF affinity columns, and fusion protein GST-LytA was further purified by thrombin according to the instructions of manufacturer (Biopharmacia Ltd.) to obtain rLytA. Pyrosate (Associates of Cape Cod Inc., ACC) test kit with a sensitivity of 60 EU/ml was used for endotoxin test, and no endotoxin was detectable in purified protein. Before used as immunogen to immunize mice, purified rLytA was quantified by Bradford assay.
Western blot analysis
Streptococcus pneumoniae serotype 23F strain SH137 was freshly collected from blood agar base and suspended in PBS. Two mice were injected at different points on the back with SH137 strain (1 × 109 CFU) twice the first week then once per week for total 6 weeks, then mice were killed. Serum was collected and diluted at 1:500 and then was used as primary antibody to perform western blot analysis according to standard protocol. In this experiment, 3 μg of purified rLytA from strain SH69, SH137, SH143, Beijing standard 31108 and purified GST protein were loaded to run SDS–PAGE.
Nasopharynx-lung-infected mice model
To build nasopharynx-lung-infected mice model, fresh cultured S. pneumoniae serotype 23F strain SH137 (3 × 108 CFU) was suspended in 20 μl of sterile PBS, then it was slowly and carefully instilled in the nostril of each experiment group mice (10 mice), and other 5 mice were given only 20 μl of sterile PBS as negative control. Five days later, blood samples of mice were bled from orbital plexus, then mice were killed, and nasopharynx lavage fluid was gathered from the tip of nose after injecting 1.5 ml of Ringer’s solution into trachea. Lung lavage fluid was also gathered by injecting Ringer’s solution into lung, and fluid was drawn back after the lung became expanded. Samples from every mouse were serially diluted, then inoculated onto Columbia blood agar plates (5 mg/l gentamicin was added to inhibit the growth of other bacteria that may present) and incubated at 37°C for 18 h. Cell clones were chosen for optochin test (5 μg/piece, bioMerieux company) to identify S. pneumoniae. Pathological analysis of lung from infected and mock-infected mice was performed by the department of pathology at Sun Yat-sen university.
Intranasal immunization, sample collection and ELISA
To build intranasal immunization model, 10 mice were instilled through nares with 6 μg rLytA (from strain SH137) together with 10 μg CpG ODN in a total volume of 20 μl, twice per week for 3 weeks. Mice were killed at 2 weeks after last immunization. As positive control, 10 mice were administered by a single injection with 10 μg of PS vaccine (23-valent polysaccharide commercial vaccine Pneumovax® 23, Guangzhou Center for Disease Control and Prevention) followed by the direction of manufacturer, and other 5 mice were instilled through nares with 10 μg CpG ODN only as negative control.
Nasopharynx wash samples and blood from mice were collected as mentioned previously, and sera were separated from blood. All samples were stored at −20°C for enzyme-linked immunosorbent assay (ELISA). Serum IgG anti-rLytA, IgA anti-rLytA, nasopharynx wash sIgA anti-rLytA and serum anti-PS IgG were measured by ELISA. Briefly, plates were coated with PS or rLytA (both were 100 μl of 5 μg/ml stock in PBS per well) at 4°C overnight, then blocked with 4% nonfat powdered milk. Serum and nasopharynx wash samples from mice were tested at a dilution of 1:200 and 1:10, respectively. Antibody binding was detected by using a 1:10,000 dilution of peroxidase-conjugated goat anti-mouse IgG or 1:4,000 dilution of peroxidase-conjugated goat anti-mouse IgA for 1 h at 37°C. Plates were developed with o-phenylenediamine dihydrochloride (Sigma Chemical Co.) and stopped with 1 M H2SO4 before reading the OD value at 492 nm.
Protective efficacy analysis
Ninety mice were equally and randomly separated into three groups: Group A were immunized through nares with rLytA from strain SH137 as described above; group B were given 10 μg of PS vaccine as above; and group C were given 20 μl of sterile saline exactly the same way as group A.
After last immunization, the 30 mice of each group were treated through 2 different schemes: (1) 20 mice were challenged by intraperitoneal injection of living S. pneumoniae strain SH137 2 weeks after the last immunization. Dosage of bacteria used was 3 × 107 CFU based on our pre-experiment (about 85% mice died by using this dosage and none of the mice got sepsis). Mice alive 14 days after challenge were considered to survive, and survival rate of each group was counted [16]. (2) The rest 10 mice of each group received intranasal infection with S. pneumoniae strain SH137 and 5 days later, nasopharyngeal and lung wash samples of those mice were collected, diluted and cultured as described above, whereafter, CFU of S. pneumoniae was counted.
Cross-protective efficacy analysis
We also repeated the above-mentioned experiment by intranasal immunization mice with rLytA from strains SH137, then intraperitoneal challenged mice with serotypes from other pathogenic S. pneumoniae strains SH69, SH84, SH90 and SH143 in order to test whether rLytA from one serotype could cross-protect infection of other strains. Dosages used for those strains were 3 × 106 CFU, 8 × 105 CFU, 104 CFU and 4 × 104 CFU, respectively.
Statistical analysis
ELISA data were calculated by Student’s t test; local protection efficacy was determined by comparing the number of pneumococcus in colon from trachea–nasopharynx lavage fluid among different mice groups, and difference was determined by Kruskal–Wallis Test; Mann–Whitney U test was used to compare mortality difference among groups. Data were considered to be significant when P value is below 0.05.
Results
Sequence analysis
Amino acid sequence analysis of full-length LytA region from six Chinese isolates was shown compared with LytA from R6 strain (Fig. 1, DNA sequence not shown). The N-terminal amidase catalytic domain and the key amino acid Ile315 in both activity and fold [17] were identical in all clinical isolates, which suggested that functional domain of LytA was highly conserved. One change His166 → Tyr in strain SH69, SH84, SH90 and one Gln179 → Lys in strain SH143 was found. In cell wall–binding repeats region, four amino acid alterations became obvious: Ile202 → Val, Gly204 → Asp, Asp246 → Glu in SH143 strain, and one change Tyr248 → Cys in SH137 strain was also found in this region. One alteration from Arg304 to Lys was detected in all isolates used in this study, and strains SH69, SH84, SH90 shared completely identical amino acid sequences. It is predicted that the region of amino acids from 10 to 167 was the conservative amidase, and region from 228 to 247 was identical to the conservative and repeated cell wall domain, whose function was to attach choline moieties to the cell envelope.
ClustalW alignment of the full length of LytA-predicted protein sequences. Deduced amino acid sequences of LytA from R6 strain, Beijing standard 31108, SH69, SH84, SH90, SH137 and SH143 were compared after DNA sequencing. Completely conserved residues were shown in gray background. Amidase catalytic domain was underlined. Cell wall (CW)-binding repeats characterized by conserved aromatic residues and glycines are boxed. Asterisks under the alignment show different amino acids
Western blot analysis
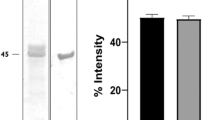
The lytA gene was highly expressed in E. coli BL21, and the expected size of the GST/rLytA fusion protein was 62 kDa including a 26-kDa GST tag from vector pGEX-4T-1, and purified rLytA protein was 36 kDa (Fig. 2a).
SDS–PAGE analysis and western blot analysis of purified rLytA. The lytA gene was highly expressed in E. coli BL21, and rLytA was purified by using GSTrap FF affinity columns and thrombin as shown in (a). M molecular markers, 1 purified GST-LytA(62kD), 2 purified GST-LytA digested by thrombin, 3 purified rLyt(36kD), 4 purified GST(26kD). Western blot analysis was performed by using serum from serotype 23F strain SH137 infected mice as primary antibody to show the cross-reacting among rLytA from different serotypes (b). M molecular markers, 1 negative control (purified GST), 2 purified rLytA from Beijing standard 31108 strain, 3 purified rLytA from serotype 19F strain SH69, 4 purified rLytA from serotype 23F strain SH137 and 5 purified rLytA from serotype 6B strain SH143
Serum from mice infected with S. pneumoniae serotype 23F strain SH137 was used as primary antibody to detect the cross-reaction among rLytA from different serotypes. Because strains SH69, SH84, SH90 had the same amino acid sequences, only purified rLytA from Beijing standard 31108 strain, SH69 strain, SH137 strain and SH143 strain were used in this study. As shown in Fig. 2b, a band at approximately 36 kDa was observed from lanes 2 to 5, suggesting that purified rLytA proteins from different serotype isolates were recognized by serum from mice infected with the 23F strain SH137. No detectable band was found in the purified GST protein control (lane 1).
Antibody production responses to immunization
To identify antibody production in mice immunized with rLytA (group A) or PS vaccine (group B), ELISAs were conducted and in order to make difference among groups more obvious, we optimized dilution of serum and nasopharynx wash samples at 1:200 and 1:10, respectively. In this experiment, what we are concerned was the difference among groups, so data were presented as values at OD492 nm instead of titer.
Intranasal immunization with rLytA elicited significant IgG and IgA antibodies in serum and sIgA in nasopharyngeal washes in group A mice; anti-PS IgG antibody in serum was detected in group B mice; no antibody was detectable in negative control group C as shown in Fig. 3. These data showed that intranasal immunization with rLytA induced both IgG and IgA in serum and sIgA in local area, which is supposed to be more effective in protection against S. pneumoniae infection.
Effect of intranasal immunization with rLytA on antibody response in mice. Three groups of mice were intranasally immunized either with rLytA (from strain SH137), with CpG ODN (group A), or single injection with PS vaccine (group B) or only CpG ODN through nares as negative control (group C). Serum and nasopharynx lavage fluid were gathered to perform ELISA to determine antibody response. Both anti-rLytA IgG and IgA in serum and anti-rLytA sIgA in nasopharynx lavage fluid were detected in group A mice, anti-PS IgG in serum was detected in group B mice, and no antibody was detected in group C mice. The mean value of antibody in each group was shown here, and asterisks indicate significance at P < 0.05
Nasopharynx-lung-infected mice model
Naturally, S. pneumoniae were colonized at nasopharyngeal area of host so we tried to establish nasopharynx-lung-infected mice model to simulate this natural situation in order to investigate intranasal immunity efficacy of rLytA as a protein vaccine.
Nasopharynx wash sample, lung wash sample and blood of S. pneumoniae infected mice were collected and cultured on Columbia blood agar plates. S. pneumoniae were detected in both nasopharynx and lung washes, but not in blood. Compared with the lung of control mice (Fig. 4a), pathological changes were significant in lung tissues of infected mice: infected areas were congested and filled with neutrophil and macrophage and apparently were in an edema state (Fig. 4b). These observations indicated that the nasopharynx-lung-infected mice model was successfully established.
Pathological analysis of lung from nasopharynx-lung-infected mice model. Mice were instilled fresh cultured S. pneumoniae serotype 23F strain SH137 through nares, then mice were killed, and lung tissue was collected for pathological analysis. Compared with lung of mock-infected mice (a), lung of infected mice b was full of neutrophils and macrophage
Protection against intraperitoneal challenge and intranasal infection
To further test whether IgG, IgA and sIgA induced by intranasal immunization with rLytA could protect mice against S. pneumoniae infection or not, intraperitoneal challenge and intranasal infection were utilized. The average number of surviving mice from two independent experiments of group A (rLytA intranasal-immunized group) group B (PS vaccine–immunized group) and group C (negative control group) were 12.5, 13 and 2.5, respectively, and survival rates of 3 groups were 62.5, 65 and 12.5%, respectively. The survival rate of group C was significantly lower than that of the other two groups (P < 0.05), and difference between group A and group B is not significant (P > 0.05) (Fig. 5a).
Effect of rLytA vaccination on survival of mice against intraperitoneal challenge and intranasal infection by S. pneumoniae. After intranasal immunization with rLytA from strain SH137 (group A) or injection immunization with PS vaccine (group B), mice were given S. pneumoniae strain SH137 by different procedures: intraperitoneal challenge then monitored for survival percent (a) or intranasal infected and then CFU of S. pneumoniae from nasopharyngeal wash samples was counted (b). Compared with negative control group C, survival percent of both group A and group B was significant higher (P < 0.05), whereas no difference between these two groups but only CFU from group A was significant lower. Asterisks indicate significance at P < 0.05
Geometric mean number CFU from nasopharynx washes of group A mice was markedly lower than that of group B mice and group C mice (P < 0.05), suggesting that much less S. pneumoniae colonized at nasopharyngeal site of group A mice (Fig. 5b). No pneumococci were cultured from lung lavage fluid from both group A and group B mice, whereas lung lavage fluid from 7 out of the 10 mice of group C contained S. pneumoniae with average 105 CFU per mouse.
Cross-protective efficacy against intraperitoneal challenge
To test cross-protection efficacy of rLytA from serotype 23F strain SH137, other four strains: serotype19F strain SH69, serotype14 strain SH84, serotype 6A strain SH90 and serotype 6B strain SH143 of S. pneumoniae were utilized to repeat intraperitoneal challenge experiment. Survival rates of rLytA (from SH137 strain)-immunized mice of the four strain groups (75, 65, 62.5 and 60%, respectively) were significantly increased compared with each of their negative control group (20, 17.5, 17.5 and 15%, respectively) (Fig. 6). The result strongly suggested that rLytA from one serotype strain may protect infection from other serotype strains.
Cross-protective efficacy of rLytA among different serotypes. After immunization with rLytA from 23F strain SH137, mice were intraperitoneally challenged with serotypes: 19F strain SH69, 6A strain SH90, 6B strain SH143 and 14 strain SH84, then survival percent was monitored. Survival rate of all groups was significantly higher than their negative control group
Discussion
Protein-based S. pneumoniae vaccines have the potential advantages of non-serotype limited protection across capsular types, comparatively inexpensive to produce by recombinant DNA techniques, and able to induce memory responses that are long-lasting and can be boosted by revaccination [3]. To be a new S. pneumoniae protein vaccine, first and foremost, structure of potential candidate protein must be conserved among different capsular serotypes and it is also a wise strategy to avoid the possible non-vaccine serotypes replacement. Second, new vaccine candidates should protect against S. pneumoniae infection and colonization of respiratory tract, which are the most common clinical syndromes associated with this pathogen. Application to mucosa is a promising route of immunization by avoiding the need for needles to administer vaccine, increasing safety and acceptability.
Today, worldwide research on this subject is the search for possible protein vaccine candidates. Controversial data had been reported regarding the protective properties of LytA [6]. We collected the most pathogenic 5 serotype isolates of S. pneumoniae from China and discussed whether LytA was antigenically conserved and whether its elicited antibodies could reduce colonization.
Our data showed that sequences encoding LytA from serotype isolates 19F (strain SH69), 14 (strain SH84), 6A (strain SH90), 23F (strain SH137) and 6B (strain SH143) were highly identical (96.75–99.79% identity). Only six substitutions as compared with R6 strain were found, and the N-terminal amidase catalytic domain and the key amino acid Ile315 were identical in all isolates. Amino acid sequencing analysis preliminarily proved that sequences of LtyA were highly conserved among different serotype isolates. Result of intercross western blot analysis also showed that all rLytAs from those 5 serotype strains recognized serum from mice infected with 23F strain SH137, implying that rLytAs may possess same antigenicity or common antigenic epitopes, thereby further suggesting that autolysin of S. pneumoniae was conserved and could be considered as a competitive candidate for protein vaccine.
In the nasopharynx-lung-infected mice model, the fact that S. pneumoniae were detectable in nasopharynx and lung lavage fluid but were absent in blood supported that pneumococci present in nasopharynx were colonized, not contaminated. Although both intranasal immunization with rLytA and intramuscular injection with PS vaccine induced systemic IgG antibody response, only the former induced anti-rLytA antibody in secretions (sIgA) and serum (IgA) as shown in ELISA data. Because sIgA is desirable to defense against mucosal infections by inhibiting initial pathogen colonization at the mucosal surface, we assumed that sIgA might decrease the carriage of S. pneumoniae. Furthermore, the anti-rLytA IgG and IgA in serum may also provide systemic protection against bacterial infection.
To prove our hypothesis that nasal inoculation of mice with rLytA could induce both systemic and local protective immunity, we measured mice survival rate after challenge and the CFU of S. pneumoniae in mice nasopharynx area. Our data showed that mice survival rate was significantly increased by intranasal immunization with rLytA or injection with PS vaccine, and there was no difference between these two groups, which indicated that protective efficiency induced by nasal inoculation with rLytA was as good as that induced by intramuscular injection with PS vaccine. Moreover, data of cross-protective efficacy showed that immunization with rLytA of one serotype strain indeed protected mice from the infection of other pathogenic strains, which indicated the non-serotype-specific protection of rLytA. The bacteria CFU at nasopharynx area of rLytA intranasal immunization group was significantly lower than that of other two groups, suggesting that protection against colonization and carriage might be the result of direct protection elicited by mucosal immunization in nasopharynx, so local rather than systemic immunity played a more important role in this case.
In conclusion, this study demonstrated that immunization of mice through nasopharynx with rLytA generated a protective immunity in both systemic and mucosal compartments, which strengthened the argument about rLytA being a vaccine candidate to test mucosal immunization as a potential route for the delivery of pneumococcal vaccines in humans. However, this study was conducted on mice model, so the knowledge of exact relevance of these data to human carriage and systemic pneumococcal disease is still unknown. Our further studies will be focused on primate non-human model and more different serotype isolates of S. pneumoniae.
References
Butler JC, Shapiro ED, Carlone GM. Pneumococcal vaccines: history, current status, and future directions. Am J Med. 1999;107:69S–76S.
Kadioglu A, Andrew PW. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 2004;25:143–9.
Swiatlo E, Ware D. Novel vaccine strategies with protein antigens of Streptococcus pneumoniae. FEMS Immunol Med Microbiol. 2003;38:1–7.
Fernandez-Tornero C, Garcia E, Lopez R, Gimenez-Gallego G, Romero A. Two new crystal forms of the choline-binding domain of the major pneumococcal autolysin: insights into the dynamics of the active homodimer. J Mol Biol. 2002;32:163–73.
Bogaert D, Hermans PW, Adrian PV, Rumke HC, de Groot R. Pneumococcal vaccines: an update on current strategies. Vaccine. 2004;22:2209–20.
Jedrzejas MJ. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev. 2001;65:187–207.
Palaniappan R, Singh S, Singh UP, Sakthivel SK, Ades EW, Briles DE, et al. Differential PsaA-, PspA-, PspC-, and PdB-specific immune responses in a mouse model of pneumococcal carriage. Infect Immun. 2005;73:1006–13.
van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005;73:7718–26.
Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Nat Med. 2003;9:99–103.
Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–58.
Zhao GM, Black S, Shinefield H, Wang CQ, Zhang YH, Lin YZ, et al. Serotype distribution and antimicrobial resistance patterns in Streptococcus pneumoniae isolates from hospitalized pediatric patients with respiratory infections in Shanghai, China. Pediatr Infect Dis J. 2003;22:739–42.
Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–6.
McCluskie MJ, Davis HL. CpG DNA as mucosal adjuvant. Vaccine. 1999;18:231–7.
O’Hagan DT, MacKichan ML, Singh M. Recent developments in adjuvants for vaccines against infectious diseases. Biomol Eng. 2001;18:69–85.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80.
Brandtzaeg P. Role of secretory antibodies in the defence against infections. Int J Med Microbiol. 2003;293:3–15.
Romero P, Lopez R, Garcia E. Key role of amino acid residues in the dimerization and catalytic activation of the autolysin LytA, an important virulence factor in Streptococcus pneumoniae. J Biol Chem. 2007;282:17729–37.
Acknowledgments
This work was supported by National Natural Science Foundation of China, (Grant No. 81072491), Natural Science Foundation of Guangdong Province, China (Grant No. 06300650) and Guangzhou foundation for science and technology, China (Grant No. 200523-E5151).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, Z.Q., Lv, Z.Y., Gan, H.Q. et al. Intranasal immunization with autolysin (LytA) in mice model induced protection against five prevalent Streptococcus pneumoniae serotypes in China. Immunol Res 51, 108–115 (2011). https://doi.org/10.1007/s12026-011-8234-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-011-8234-x