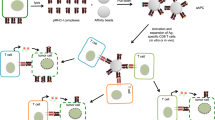
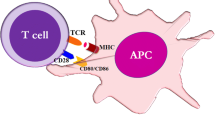
Abstract
Recent advances in molecular medicine have shown that soluble MHC-multimers can be valuable tools for both analysis and modulation of antigen-specific immune responses in vitro and in vivo. In this review, we describe the use of dimeric human and mouse major histocompatibility complexes, MHC-Ig, as part of an artificial Antigen-Presenting Cell (aAPC). MHC-Ig-based aAPC and its derivatives represent an exciting new platform technology for measuring and manipulating immune responses in vitro as well as in vivo. This new technology has the potential to help overcome many of the obstacles associated with limitations in current antigen-specific approaches of immunotherapy for the treatment of cancer, infectious diseases and autoimmunity.

Similar content being viewed by others
References
Corr M, et al. T cell receptor-MHC class I peptide interactions: affinity, kinetics, and specificity. Science. 1994;265:946–9.
Boniface JJ, et al. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands [corrected]. Immunity. 1998;9:459–66.
Greten TF, et al. Direct visualization of antigen-specific T cells: HTLV-1 Tax11–19- specific CD8(+) T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc Natl Acad Sci USA. 1998;95:7568–73.
Altman JD, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6.
Howard MC, Spack EG, Choudhury K, Greten TF, Schneck JP. MHC-based diagnostics and therapeutics—clinical applications for disease-linked genes. Immunol Today. 1999;20:161–5.
Greten TF, Schneck JP. Development and use of multimeric major histocompatibility complex molecules. Clin Diagn Lab Immunol. 2002;9:216–20.
Bercovici N, Duffour MT, Agrawal S, Salcedo M, Abastado JP. New methods for assessing T-cell responses. Clin Diagn Lab Immunol. 2000;7:859–64.
Whiteside TL. Monitoring of antigen-specific cytolytic T lymphocytes in cancer patients receiving immunotherapy. Clin Diagn Lab Immunol. 2000;7:327–32.
Oelke M, et al. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–25.
Oelke M, Krueger C, Giuntoli RL, Schneck JP. Artificial antigen-presenting cells: artificial solutions for real diseases. Trends Mol Med. 2005;11:412–20.
Oelke M, Krueger C, Schneck JP. Technological advances in adoptive immunotherapy. Drugs Today (Barc). 2005;41:13–21.
Walter EA, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44.
Heslop HE, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–5.
Aebersold P, et al. Lysis of autologous melanoma cells by tumor-infiltrating lymphocytes: association with clinical response. J Natl Cancer Inst. 1991;83:932–7.
Berthier-Vergnes O, et al. Human melanoma cells inhibit the earliest differentiation steps of human Langerhans cell precursors but failed to affect the functional maturation of epidermal Langerhans cells. Br J Cancer. 2001;85:1944–51.
Enk AH, Jonuleit H, Saloga J, Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer. 1997;73:309–16.
Valmori D, et al. Optimal activation of tumor-reactive T cells by selected antigenic peptide analogues. Int Immunol. 1999;11:1971–80.
Costa GL, et al. Adoptive immunotherapy of experimental autoimmune encephalomyelitis via T cell delivery of the IL-12 p40 subunit. J Immunol. 2001;167:2379–87.
Meidenbauer N, et al. Survival and tumor localization of adoptively transferred melan-a-specific T cells in melanoma patients. J Immunol. 2003;170:2161–9.
Brentjens RJ, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–86.
Dudley ME, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–73.
Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15.
Mocellin S, Mandruzzato S, Bronte V, Lise M, Nitti D. Part I: vaccines for solid tumours. Lancet Oncol. 2004;5:681–9.
Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–52.
Sotomayor EM, et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98:1070–7.
Lyman MA, et al. The fate of low affinity tumor-specific CD8+T cells in tumor-bearing mice. J Immunol. 2005;174:2563–72.
Woo EY, et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6.
Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66.
Ugel S, et al. In vivo administration of artificial antigen-presenting cells activates low-avidity T cells for treatment of cancer. Cancer Res. 2009;69:9376–84.
Ndhlovu ZM, et al. Development of an artificial-antigen-presenting-cell-based assay for the detection of low-frequency virus-specific CD8(+) T cells in whole blood, with application for measles virus. Clin Vaccine Immunol. 2009;16:1066–73.
Whiteside TL, et al. Enzyme-linked immunospot, cytokine flow cytometry, and tetramers in the detection of T-cell responses to a dendritic cell-based multipeptide vaccine in patients with melanoma. Clin Cancer Res. 2003;9:641–9.
Clay TM, Hobeika AC, Mosca PJ, Lyerly HK, Morse MA. Assays for monitoring cellular immune responses to active immunotherapy of cancer. Clin Cancer Res. 2001;7:1127–35.
Maecker HT, et al. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods. 2001;255:27–40.
Schutz C, et al. Killer-artificial-antigen-presenting-cells (KaAPC): a novel strategy to delete specific T cells. Blood. 2008;111(7):3546–52.
Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–88.
Chang DH, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–17.
Ishikawa A, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–7.
Osada T, Morse MA, Lyerly HK, Clay TM. Ex vivo expanded human CD4+ regulatory NKT cells suppress expansion of tumor antigen-specific CTLs. Int Immunol. 2005;17:1143–55.
Kawano T, et al. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999;59:5102–5.
Tahir SM, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–50.
Fujii S, et al. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. 2003;122:617–22.
Singh AK, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–11.
Hammond KJ, et al. CD1d-restricted NKT cells: an interstrain comparison. J Immunol. 2001;167:1164–73.
Gombert JM, et al. Early quantitative and functional deficiency of NK1+ -like thymocytes in the NOD mouse. Eur J Immunol. 1996;26:2989–98.
Webb TJ, Bieler JG, Schneck JP, Oelke M. Ex vivo induction and expansion of natural killer T cells by CD1d1-Ig coated artificial antigen presenting cells. J Immunol Methods. 2009;346:38–44.
Latouche JB, Sadelain M. Induction of human cytotoxic T lymphocytes by artificial antigen-presenting cells. Nat Biotechnol. 2000;18:405–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oelke, M., Schneck, J.P. Overview of a HLA-Ig based “Lego-like system” for T cell monitoring, modulation and expansion. Immunol Res 47, 248–256 (2010). https://doi.org/10.1007/s12026-009-8156-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-009-8156-z