Abstract
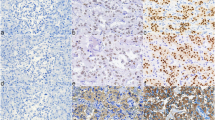
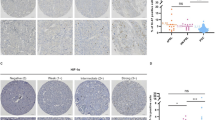
Hand2 is a core transcription factor responsible for chromaffin cell differentiation. However, its potential utility in surgical pathology has not been studied. Thus, we aimed to investigate its expression in paragangliomas, other neuroendocrine neoplasms (NENs), and additional non-neuroendocrine tumors. We calibrated Hand2 immunohistochemistry on adrenal medulla cells and analyzed H-scores in 46 paragangliomas (PGs), 9 metastatic PGs, 21 cauda equina neuroendocrine tumors (CENETs), 48 neuroendocrine carcinomas (NECs), 8 olfactory neuroblastomas (ONBs), 110 well-differentiated NETs (WDNETs), 10 adrenal cortical carcinomas, 29 adrenal cortical adenomas, 8 melanomas, 41 different carcinomas, and 10 gastrointestinal stromal tumors (GISTs). Both tissue microarrays (TMAs) and whole sections (WSs) were studied. In 171 NENs, previously published data on Phox2B and GATA3 were correlated with Hand2. Hand2 was positive in 98.1% (54/55) PGs, but only rarely in WDNETs (9.6%, 10/104), CENETs (9.5%, 2/21), NECs (4.2%, 2/48), or ONBs (12.5%, 1/8). Any Hand2 positivity was 98.1% sensitive and 91.7% specific for the diagnosis of PG. The Hand2 H-score was significantly higher in primary PGs compared to Hand2-positive WDNETs (median 166.3 vs. 7.5; p < 0.0001). Metastatic PGs were positive in 88.9% (8/9). No Hand2 positivity was observed in any adrenal cortical neoplasm or other non-neuroendocrine tumors, with exception of 8/10 GISTs. Parasympathetic PGs showed a higher Hand2 H-score compared to sympathetic PGs (median H-scores 280 vs. 104, p < 0.0001). Hand2 positivity in NENs serves as a reliable marker of primary and metastatic PG, since other NENs only rarely exhibit limited Hand2 positivity.




Similar content being viewed by others
Data Availability
The immunohistochemical results of individual tumors, together with basic clinical data, are available as Supplementary material. Additional clinical data and histological images (in the form of “*.svs” formatted image files) used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Mete O, Asa SL, Gill AJ, Kimura N, de Krijger RR, Tischler A Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas. Endocr Pathol 33: 90-114, 2022.
Miettinen M, McCue PA, Sarlomo-Rikala M et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol 38: 13-22, 2014.
Mamilla D, Manukyan I, Fetsch PA, Pacak K, Miettinen M Immunohistochemical distinction of paragangliomas from epithelial neuroendocrine tumors-gangliocytic duodenal and cauda equina paragangliomas align with epithelial neuroendocrine tumors. Hum Pathol 103: 72-82, 2020.
Kimura N, Shiga K, Kaneko K, Sugisawa C, Katabami T, Naruse M The Diagnostic Dilemma of GATA3 Immunohistochemistry in Pheochromocytoma and Paraganglioma. Endocr Pathol 31: 95-100, 2020.
Mete O, Kefeli M, Caliskan S, Asa SL GATA3 immunoreactivity expands the transcription factor profile of pituitary neuroendocrine tumors. Mod Pathol 32: 484-489, 2019.
Uccella S, Facco C, Chiaravalli AM et al. Transcription Factor Expression in Sinonasal Neuroendocrine Neoplasms and Olfactory Neuroblastoma (ONB): Hyams' Grades 1-3 ONBs Expand the Spectrum of SATB2 and GATA3-Positive Neoplasms. Endocr Pathol 33: 264-273, 2022.
Soukup J, Manethova M, Kohout A et al. Cauda equina neuroendocrine tumors show biological features distinct from other paragangliomas and visceral neuroendocrine tumors. Virchows Arch, 2022.
Xu L, Yu G, Jiang L et al. HepPar1 and GATA-3 Expression in Neuroendocrine Neoplasms: A Potential Trap for Pathologic Diagnosis. Appl Immunohistochem Mol Morphol 31: 668-672, 2023.
Perrino CM, Ho A, Dall CP, Zynger DL Utility of GATA3 in the differential diagnosis of pheochromocytoma. Histopathology 71: 475-479, 2017.
Haraguchi T, Miyoshi H, Hiraoka K et al. GATA3 Expression Is a Poor Prognostic Factor in Soft Tissue Sarcomas. PLoS One 11: e0156524, 2016.
Asa SL, Mete O Cytokeratin Profiles in Pituitary Neuroendocrine Tumors. Hum Pathol, 2020.
Manethova M, Gerykova L, Faistova H et al. Phox2B is a sensitive and reliable marker of paraganglioma-Phox2B immunohistochemistry in diagnosis of neuroendocrine neoplasms. Virchows Arch, 2023.
Miyauchi M, Akashi T, Furukawa A et al. PHOX2B is a Sensitive and Specific Marker for the Histopathological Diagnosis of Pheochromocytoma and Paraganglioma. Endocr Pathol, 2022.
Tamura M, Amano T, Shiroishi T The Hand2 gene dosage effect in developmental defects and human congenital disorders. Curr Top Dev Biol 110: 129-152, 2014.
Huber K, Karch N, Ernsberger U, Goridis C, Unsicker K The role of Phox2B in chromaffin cell development. Dev Biol 279: 501-508, 2005.
Rohrer H Transcriptional control of differentiation and neurogenesis in autonomic ganglia. Eur J Neurosci 34: 1563-1573, 2011.
Unsicker K, Huber K, Schober A, Kalcheim C Resolved and open issues in chromaffin cell development. Mech Dev 130: 324-329, 2013.
Furlan A, Dyachuk V, Kastriti ME et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 357, 2017.
Huber K, Narasimhan P, Shtukmaster S, Pfeifer D, Evans SM, Sun Y The LIM-Homeodomain transcription factor Islet-1 is required for the development of sympathetic neurons and adrenal chromaffin cells. Dev Biol 380: 286–298, 2013.
Wildner H, Gierl MS, Strehle M, Pla P, Birchmeier C Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development 135: 473-481, 2008.
Xu M, Sun M, Zhang X et al. HAND2 Assists MYCN Enhancer Invasion to Regulate a Noradrenergic Neuroblastoma Phenotype. Cancer Res 83: 686-699, 2023.
Jones A, Teschendorff AE, Li Q et al. Role of DNA methylation and epigenetic silencing of HAND2 in endometrial cancer development. PLoS Med 10: e1001551, 2013.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45: W98-W102, 2017.
Soukup J, Manethova M, Faistova H et al. Pitx2 is a useful marker of midgut-derived neuroendocrine tumours - an immunohistochemical study of 224 cases. Histopathology 81: 799-807, 2022.
Fedchenko N, Reifenrath J Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn Pathol 9: 221, 2014.
Fishbein L, Leshchiner I, Walter V et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 31: 181-193, 2017.
Akkuratova N, Faure L, Kameneva P, Kastriti ME, Adameyko I Developmental heterogeneity of embryonic neuroendocrine chromaffin cells and their maturation dynamics. Front Endocrinol (Lausanne) 13: 1020000, 2022.
Morikawa Y, D'Autreaux F, Gershon MD, Cserjesi P Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol 307: 114-126, 2007.
Hockman D, Adameyko I, Kaucka M et al. Striking parallels between carotid body glomus cell and adrenal chromaffin cell development. Dev Biol 444 Suppl 1: S308-S324, 2018.
Lee JP, Hung YP, O'Dorisio TM, Howe JR, Hornick JL, Bellizzi AM Examination of PHOX2B in adult neuroendocrine neoplasms reveals relatively frequent expression in phaeochromocytomas and paragangliomas. Histopathology 71: 503-510, 2017.
Zethoven M, Martelotto L, Pattison A et al. Single-nuclei and bulk-tissue gene-expression analysis of pheochromocytoma and paraganglioma links disease subtypes with tumor microenvironment. Nat Commun 13: 6262, 2022.
Ferguson CA, Firulli BA, Zoia M, Osterwalder M, Firulli AB Identification and characterization of Hand2 upstream genomic enhancers active in developing stomach and limbs. Dev Dyn, 2023.
Voth H, Oberthuer A, Simon T, Kahlert Y, Berthold F, Fischer M Co-regulated expression of HAND2 and DEIN by a bidirectional promoter with asymmetrical activity in neuroblastoma. BMC Mol Biol 10: 28, 2009.
Drabent P, Bonnard A, Guimiot F, Peuchmaur M, Berrebi D PHOX2B Immunostaining: A Simple and Helpful Tool for the Recognition of Ganglionic Cells and Diagnosis of Hirschsprung Disease. Am J Surg Pathol 44: 1389-1397, 2020.
Acknowledgements
We would like to thank Dr. Boris Rychly (Unilabs sro., Bratislava, SK), Dr. Marketa Trnkova (AeskuLab k.s., Prague, CR), Dr. Ludmila Michnova (Military University Hospital Prague, Prague, CR), Maria Wozniakova (University Hospital Ostrava and Faculty of Medicine, University of Ostrava), Dr. Lukas Krbal, and Dr. Hana Faistova (University Hospital Hradec Kralove) for their participation in constructing the dataset of the NENs. We would like to thank Mrs. Svetlana Kopecka, Mrs. Petra Satori, Mrs. Jana Potocna, and Mr. Michal Valasek for their assistance with immunohistochemistry procedures.
Funding
This study was supported by the project BBMRI-CZ LM2023033, Charles University Cooperatio Program, research area DIAG and METD and Project of Czech Ministry of Defense MO 1012. The work was supported by the European Regional Development Fund-Project BBMRI-CZ Biobank network—a versatile platform for the research of the etiopathogenesis of diseases, No: EF16_013/0001674. Supported by Ministry of Health, Czech Republic—conceptual development of research organization 00064165, General University Hospital in Prague and by Ministry of Health of the Czech Republic, grant NU23-01-00323 and RVO VFN64165, the Czech Science Foundation grant 22-07091S. Funding sources were not involved in study design, collection, analysis, or interpretation, in the writing of the report, or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
JS, FG, and AR conceived and designed the study; JS, JD, ISZ, MM, MN, and MH procured and reviewed the cases included in the study; TC and DN procured clinical data from tumors and histological material from CENET and helped with study design; MK, TZ, and FG procured clinical data; HH implemented, validated, and performed immunohistochemical procedures; MM, JS, FG, and VS analyzed the results of the study; JS and MM performed statistical analysis. All authors contributed to the writing and review of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The study was carried out in accordance with the Declaration of Helsinki and with the approval of the Ethics committee of the University Hospital Hradec Kralove (reference no. 202101P06 and no. 202109P01).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soukup, J., Manethova, M., Stejskal, V. et al. Hand2 Immunohistochemistry in the Diagnosis of Paragangliomas and Other Neuroendocrine Neoplasms. Endocr Pathol 35, 14–24 (2024). https://doi.org/10.1007/s12022-024-09803-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-024-09803-6