Abstract
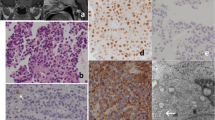
The functional differentiation of pituitary cells and adenomas follows the combination of transcription factors and co-factors in three cell lineages [growth hormone–prolactin–thyroid-stimulating hormone lineage, adrenocorticotrophic hormone (ACTH)/pro-opiomelanocortin (POMC) lineage, and follicular stimulating hormone (FSH)/luteinizing hormone (LH) lineage], which include Pit-1, GATA-2, SF-1, NeuroD1/beta2, Tpit, ERα, and others. Only rarely are hormones from different lineages co-expressed in the same adenoma cells. Most corticotroph cell adenomas belonging to the ACTH/POMC lineage are mono-hormonal. In our study of 89 corticotroph cell adenomas, 5 cases expressed both ACTH and alpha-subunit; these adenomas did not express any other anterior pituitary hormones or subunits. To clarify the mechanism involved, we studied the transcription factors that regulate pituitary cell differentiation. NeuroD1 and T-pit, markers of the ACTH/POMC lineage, and SF-1 and DAX-1, related to the LH/FSH cell lineage were expressed in all cases. GATA2, a synergistic factor in the gonadotroph cell lineage with SF-1, was also expressed in three of five cases. As ACTH and alpha-subunit are the earliest hormones to appear during development, we speculate that these particular adenomas are derived from committed ACTH progenitor cells. The molecular process governing functional differentiation of these adenomas requires further investigation.







Similar content being viewed by others
References
Asa SL, Bamberger AM, Cao B, Wong M, Parker KL, Ezzat S. The transcription activator steroidogenic factor-1 is preferentially expressed in the human pituitary gonadotroph. J Clin Endocrinol Metab 81:2165–70, 1996.
Asa SL, Puy LA, Lew AM, Sundmark VC, Elsholtz HP. Cell type-specific expression of the pituitary transcription activator pit-1 in the human pituitary and pituitary adenomas. J Clin Endocrinol Metab 77:1275–80, 1993.
Aylwin SJ, Welch JP, Davey CL, Geddes JF, Wood DF, Besser GM, et al. The relationship between steroidogenic factor 1 and DAX-1 expression and in vitro gonadotropin secretion in human pituitary adenomas. J Clin Endocrinol Metab 86:2476–83, 2001.
Ferretti E, Di Stefano D, Zazzeroni F, Gallo R, Fratticci A, Carfagnini R, et al. Human pituitary tumours express the bHLH transcription factors NeuroD1 and ASH1. J Endocrinol Invest 26:957–65, 2003.
Friend KE, Chiou YK, Lopes MB, Laws ER Jr, Hughes KM, Shupnik MA. Estrogen receptor expression in human pituitary: correlation with immunohistochemistry in normal tissue, and immunohistochemistry and morphology in macroadenomas. J Clin Endocrinol Metab 78:1497–504, 1994.
Ikuyama S, Mu YM, Ohe K, Nakagaki H, Fukushima T, Takayanagi R, et al. Expression of an orphan nuclear receptor DAX-1 in human pituitary adenomas. Clin Endocrinol (Oxf) 48:647–54, 1998.
La Rosa S, Celato N, Uccella S, Capella C. Detection of gonadotropin-releasing hormone receptor in normal human pituitary cells and pituitary adenomas using immunohistochemistry. Virchows Arch 437:264–9, 2000.
Lamolet B, Poulin G, Chu K, Guillemot F, Tsai MJ, Drouin J. Tpit-independent function of NeuroD1(BETA2) in pituitary corticotroph differentiation. Mol Endocrinol 18:995–1003, 2004.
Matsuno A, Katakami H, Nagashima T, Teramoto A, Osamura RY, Kirino T. Growth hormone-releasing hormone expression in pituitary somatotroph adenomas, studied by immunohistochemistry and in situ hybridization using catalyzed signal amplification system. Hum Pathol 31:789–94, 2000.
Miller GM, Alexander JM, Klibanski A. Gonadotropin-releasing hormone messenger RNA expression in gonadotroph tumors and normal human pituitary. J Clin Endocrinol Metab 81:80–3, 1996.
Oyama K, Sanno N, Teramoto A, Osamura RY. Expression of neuro D1 in human normal pituitaries and pituitary adenomas. Mod Pathol 14:892–9, 2001.
Sanno N, Teramoto A, Matsuno A, Itoh J, Takekoshi S, Osamura RY. In situ hybridization analysis of Pit-1 mRNA and hormonal production in human pituitary adenomas. Acta Neuropathol (Berl) 91:263–8, 1996.
Umeoka K, Sanno N, Osamura RY, Teramoto A. Expression of GATA-2 in human pituitary adenomas. Mod Pathol 15:11–7, 2002.
Zafar M, Ezzat S, Ramyar L, Pan N, Smyth HS, Asa SL. Cell-specific expression of estrogen receptor in the human pituitary and its adenomas. J Clin Endocrinol Metab 80:3621–7, 1995.
Osamura RY, Watanabe K. Immunohistochemical studies of human FSH producing pituitary adenomas. Virchows Arch A Pathol Anat Histopathol 413:61–8, 1988.
Sano T, Kovacs K, Asa SL, Smyth HS. Immunoreactive luteinizing hormone in functioning corticotroph adenomas of the pituitary. Immunohistochemical and tissue culture studies of two cases. Virchows Arch A Pathol Anat Histopathol 417:361–7, 1990.
Egensperger R, Scheithauer BW, Horvath E, Kovacs K, Giannini C, Young WF, et al. Cushing’s disease due to plurihormonal adrenocorticotropic hormone and gonadotropin-producing pituitary adenoma. Acta Neuropathol (Berl) 102:398–403, 2001.
Ikeda H, Yoshimoto T, Kovacs K, Horvath E. Cushing’s disease due to female gonadotroph adenoma of the pituitary. Clin Endocrinol (Oxf) 43:383–6, 1995.
Osamura RY. Functional prenatal development of anencephalic and normal anterior pituitary glands. In human and experimental animals studied by peroxidase-labeled antibody method. Acta Pathol Jpn 27(4):495–509, 1977.
Asa SL, Kavacs K, Laszlo FA, Domokos I, Ezrin C. Human fetal adenohypophysis histogeic and immunocytochemical analysis. Neuroendocrinology 43(3):308–16, 1986.
Berg KK, Scheithauer BW, Felix I, Kovacs K, Horvath E, Klee GG, et al. Pituitary adenomas that produce adrenocorticotropic hormone and alpha-subunit: clinicopathological, immunohistochemical, ultrastructural, and immunoelectron microscopic studies in nine cases. Neurosurgery 26:397–403, 1990.
Desai B, Burrin JM, Nott CA, Geddes JF, Lamb EJ, Aylwin SJ, et al. Glycoprotein hormone alpha-subunit production and plurihormonality in human corticotroph tumours—an in vitro and immunohistochemical study. Eur J Endocrinol 133:25–32, 1995.
Osamura RY, Watanabe K. Immunohistochemical colocalization of growth hormone (GH) and α subunit in human GH secreting pituitary adenomas. Virchows Archiv A 411:323–30, 1987.
Xu B, Sano A, Yamada S, Li CC, Hirokawa M. Expression of corticotrophin-releasing hormone messenger ribonucleic acid in human pituitary corticotroph adenomas associated with proliferative potential. J Clin Endocrinol Metab 85(3):1220–5, 2000.
Losa M, Barzanghi RL, Mortini P, Franzin A, Mangili F, Terreni MR, et al. Determination of the proliferation and apoptotic index in adrenocorticotropin-secreting pituitary tumors: comparison between micro- and macroadenomas. Am J Pathol 156(1):245–51, 2000.
Mastronardi L, Guiducci A, Spera C, Puzzilli F, Liberati F, Ruggeri A, et al. Adrenocorticotropic hormone secreting pituitary adenomas: analysis of growth fraction using the MIB-1 antibody. Tumori 86(3):229–32, 2000.
Mastronardi L, Guiducci A, Spera C, Puzzilli F, Liberati F, Maira G. Ki-67 labelling index and invasiveness among anterior pituitary adenomas: analysis of 103 cases using the MIB-1 monoclonal antibody. J Clin Pathol 52(2):107–11, 1999.
Tahara S, Kurotani R, Ishii Y, Sanno N, Teramoto A, Osamura RY. A case of Cushing’s disease caused by pituitary adenoma producing adrenocorticotropic hormone and growth hormone concomitantly: aberrant expression of transcription factors NeuroD1 and Pit-1 as a proposed mechanism. Mod Pathol 15(10):1102–5, 2002.
Kurotani R, Yoshimura S, Iwasaki Y, Inoue K, Teramoto A, Osamura RY. Exogenous expression of Pit-1 in AtT-20 corticotropic cells induces endogenous growth hormone gene transcription. J Endocrinol 172:477–87, 2002.
Barnhart KM, Mellon PL. The orphan nuclear receptor, steroidogenic factor-1, regulates the glycoprotein hormone alpha-subunit gene in pituitary gonadotropes. Mol Endocrinol 8:878–85, 1994.
Osamura RY, Tahara S, Komatsubara K, Itoh Y, Kajiwara H, Kurotani R, et al. Pit-1 positive alpha-subunit positive nonfunctioning human pituitary adenomas: a dedifferentiated GH cell lineage? Pituitary 1:269–71, 1999.
Delegeane AM, Ferland LH, Mellon P. Tissue specific enhancer of the human glycoprotein hormone a subunit gene: dependence on cAMP inducible elements. Mol Cell Biol 7:3994–4002, 1987.
Pulichino AM, Vallette Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev 17:738–47, 2003.
Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev Biol 265(2):329–40, 2004.
Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol 20(11):2898–908, 2006.
Ishii Y, Suzuki M, Takekoshi S, Egashira N, Yamazaki M, Miyai S, et al. Immunonegative “null cell” adenomas and gonadotropin (Gn) subunit (SUs) immunopositive adenomas share frequent expression of multiple transcription factors. Endocr Pathol 17:35–43, 2006.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research Projects (B, 16390110 and 16790836) of the Ministry of Education Culture, Sports, Science and Technology, Japan, and by the Research on Measures for Intractable Diseases Project of the Hypothalamo-Pituitary Dysfunction Research Group of the Ministry of Health, Labor and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, M., Egashira, N., Kajiya, H. et al. ACTH and α-Subunit are Co-expressed in Rare Human Pituitary Corticotroph Cell Adenomas Proposed to Originate from ACTH-Committed Early Pituitary Progenitor Cells. Endocr Pathol 19, 17–26 (2008). https://doi.org/10.1007/s12022-008-9014-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-008-9014-6