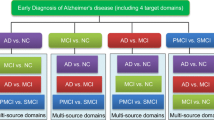
Abstract
Recently, transfer learning has been successfully applied in early diagnosis of Alzheimer’s Disease (AD) based on multi-domain data. However, most of existing methods only use data from a single auxiliary domain, and thus cannot utilize the intrinsic useful correlation information from multiple domains. Accordingly, in this paper, we consider the joint learning of tasks in multi-auxiliary domains and the target domain, and propose a novel Multi-Domain Transfer Learning (MDTL) framework for early diagnosis of AD. Specifically, the proposed MDTL framework consists of two key components: 1) a multi-domain transfer feature selection (MDTFS) model that selects the most informative feature subset from multi-domain data, and 2) a multi-domain transfer classification (MDTC) model that can identify disease status for early AD detection. We evaluate our method on 807 subjects from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database using baseline magnetic resonance imaging (MRI) data. The experimental results show that the proposed MDTL method can effectively utilize multi-auxiliary domain data for improving the learning performance in the target domain, compared with several state-of-the-art methods.





Similar content being viewed by others
Notes
P ∈ {0.000001, 0.00001, 0.0001, 0.0003, 0.0007, 0.001, 0.003, 0.005, 0.007, 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9}
References
Association, A.s (2014). 2014 Alzheimer’s disease facts and figures. Alzheimers Dement, 10, 47–92.
Chang, C.C., Lin, C.J., (2001). LIBSVM: a library for support vector machines http://www.csie.ntu.edu.tw/~cjlin/libsvm/ .
Chao, L. L., Buckley, S. T., Kornak, J., Schuff, N., Madison, C., Yaffe, K., Miller, B. L., Kramer, J. H., & Weiner, M. W. (2010). ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Disease and Associated Disorders, 24, 19–27.
Chen, X., Pan, W., Kwok, J. T., & Carbonell, J. G. (2009). Accelerated gradient method for multi-task sparse learning problem. In Proceeding of ninth IEEE international conference on data mining and knowledge discovery (pp. 746–751).
Cheng, B., Zhang, D., & Shen, D. (2012). Domain transfer learning for MCI conversion prediction. In Proceeding of International Conference on Medical Image Computing and Computer-Assisted Intervention-MICCAI 2012 7510 (pp. 82–90).
Cheng, B., Zhang, D., Chen, S., Kaufer, D. I., Shen, D., & ADNI (2013). Semi-supervised multimodal relevance vector regression improves cognitive performance estimation from imaging and biological biomarkers. Neuroinformatics, 11, 339–353.
Cheng, B., Liu, M., Suk, H., Shen, D., & Zhang, D. (2015a). Multimodal manifold-regularized transfer learning for MCI conversion prediction. Brain Imaging and Behavior, 9, 913–926.
Cheng, B., Liu, M., Zhang, D., Munsell, B. C., & Shen, D. (2015b). Domain transfer learning for MCI conversion prediction. IEEE Transactions on Biomedical Engineering, 62, 1805–1817.
Chetelat, G., Landeau, B., Eustache, F., Mezenge, F., Viader, F., de la Sayette, V., Desgranges, B., & Baron, J. C. (2005). Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. NeuroImage, 27, 934–946.
Cho, Y., Seong, J. K., Jeong, Y., Shin, S. Y., & ADNI (2012). Individual subject classification for Alzheimer’s disease based on incremental learning using a spatial frequency representation of cortical thickness data. NeuroImage, 59, 2217–2230.
CIT, (2012). Medical Image Processing, Analysis and Visualization (MIPAV) http://mipav.cit.nih.gov/clickwrap.php .
Coupé, P., Eskildsen, S. F., Manjón, J. V., Fonov, V. S., Pruessner, J. C., Allard, M., & Collins, D. L. (2012). Scoring by nonlocal image patch estimator for early detection of Alzheimer’s disease. NeuroImage: Clinical, 1, 141–152.
Cuingnet, R., Gerardin, E., Tessieras, J., Auzias, G., Lehericy, S., Habert, M. O., Chupin, M., Benali, H., & Colliot, O. (2011). Automatic classification of patients with Alzheimer’s disease from structural MRI: a comparison of ten methods using the ADNI database. NeuroImage, 56, 766–781.
Da, X., Toledo, J. B., Zee, J., Wolk, D. A., Xie, S. X., Ou, Y., Shacklett, A., Parmpi, P., Shaw, L., Trojanowski, J. Q., & Davatzikos, C. (2014). Integration and relative value of biomarkers for prediction of MCI to AD progression: spatial patterns of brain atrophy, cognitive scores, APOE genotype and CSF biomarkers. NeuroImage: Clinical, 4, 164–173.
Davatzikos, C., Bhatt, P., Shaw, L.M., Batmanghelich, K.N., Trojanowski, J.Q., (2011). Prediction of MCI to AD conversion, via MRI, CSF biomarkers, and pattern classification. Neurobiology of Aging 32, 2322.e2319–2322.e2327.
DeLong, E. R., DeLong, D. M., & Clarke-Pearson, D. L. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics, 44, 837–845.
Duan, L. X., Tsang, I. W., & Xu, D. (2012). Domain transfer multiple kernel learning. IEEE Transactions on Pattern Analysis and Machine Intelligence, 34, 465–479.
Duchesne, S., & Mouiha, A. (2011). Morphological factor estimation via high-dimensional reduction: prediction of MCI conversion to probable AD. International Journal of Alzheimer's Disease, 2011, 914085.
Eskildsen, S. F., Coupé, P., García-Lorenzo, D., Fonov, V., Pruessner, J. C., & Collins, D. L. (2013). Prediction of Alzheimer’s disease in subjects with mild cognitive impairment from the ADNI cohort using patterns of cortical thinning. NeuroImage, 65, 511–521.
Fan, Y., Batmanghelich, N., Clark, C. M., Davatzikos, C., & Initia, A. D. N. (2008). Spatial patterns of brain atrophy in MCI patients, identified via high-dimensional pattern classification, predict subsequent cognitive decline. NeuroImage, 39, 1731–1743.
Filipovych, R., Davatzikos, C., & Initia, A. D. N. (2011). Semi-supervised pattern classification of medical images: application to mild cognitive impairment (MCI). NeuroImage, 55, 1109–1119.
Gaser, C., Franke, K., Kloppel, S., Koutsouleris, N., & Sauer, H. (2013). BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease. PloS One, 8, e67346.
Guo, X., Wang, Z., Li, K., Li, Z., Qi, Z., Jin, Z., Yao, L., & Chen, K. (2010). Voxel-based assessment of gray and whitematter volumes in Alzheimer’s disease. Neuroscience Letters, 468, 146–150.
Hinrichs, C., Singh, V., Xu, G. F., Johnson, S. C., & Neuroimaging, A. D. (2011). Predictive markers for AD in a multi-modality framework: an analysis of MCI progression in the ADNI population. NeuroImage, 55, 574–589.
Hu, K., Wang, Y., Chen, K., Hou, L., & Zhang, X. (2016). Multi-scale features extraction from baseline structure MRI for MCI patient classification and AD early diagnosis. Neurocomputing, 175, 132–145.
Jie, B., Zhang, D., Cheng, B., & Shen, D. (2015). Manifold regularized multitask feature learning for multimodality disease classification. Human Brain Mapping, 36, 489–507.
Kabani, N., MacDonald, D., Holmes, C. J., & Evans, A. (1998). A 3D atlas of the human brain. NeuroImage, 7, S717.
Khedher, L., Ramírez, J., Górriz, J. M., Brahim, A., & Segovia, F. (2015). Early diagnosis of Alzheimer’s disease based on partial least squares, principal component analysis and support vector machine using segmented MRI images. Neurocomputing, 151, 139–150.
Li, H., Liu, Y., Gong, P., Zhang, C., & Ye, J. (2014). Hierarchical interactions model for predicting mild cognitive impairment (MCI) to Alzheimer’s disease (AD) conversion. PloS One, 9, e82450.
Liu, J., Ji, S., Ye, J., (2009). SLEP: sparse learning with efficient projections. Arizona State University, http://www.public.asu.edu/~jye02/Software/SLEP .
Liu, F., Wee, C. Y., Chen, H. F., Shen, D. G., & ADNI (2014). Inter-modality relationship constrained multi-modality multi-task feature selection for Alzheimer’s disease and mild cognitive impairment identification. NeuroImage, 84, 466–475.
Liu, M., Zhang, D., & Shen, D., (2016a). Inherent structure based multi-view learning with multi-atlas feature representation for alzheimer's disease diagnosis. IEEE Transactions on Biomedical Engineering, 63, 1473–1482.
Liu, M., Zhang, D., & Shen, D., (2016b). Relationship induced multi-template learning for diagnosis of alzheimer's disease and mild cognitive impairment. IEEE Transactions on Medical Imaging, 35, 1463–1474.
Misra, C., Fan, Y., & Davatzikos, C. (2009). Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: results from ADNI. NeuroImage, 44, 1415–1422.
Moradi, E., Pepe, A., Gaser, C., Huttunen, H., & Tohka, J. (2015). Machine learning framework for early MRI-based Alzheimer’s conversion prediction in MCI subjects. NeuroImage, 104, 398–412.
Nemirovski, A., (2005). Efficient method s in convex programming.
Obozinski, G., Taskar, B., & Jordan, M. I. (2006). Multi-task feature selection. Statistics Department, UC Berkeley: Technical report.
Ota, K., Oishi, N., Ito, K., Fukuyama, H., & Grp, S.-J. S. (2014). A comparison of three brain atlases for MCI prediction. Journal of Neuroscience Methods, 221, 139–150.
Ota, K., Oishi, N., Ito, K., & Fukuyama, H. (2015). Effects of imaging modalities, brain atlases and feature selection on prediction of Alzheimer’s disease. Journal of Neuroscience Methods, 256, 168–183.
Pan, S. J., & Yang, Q. (2010). A survey on transfer learning. IEEE Transactions on Knowledge and Data Engineering, 22, 1345–1359.
Querbes, O., Aubry, F., Pariente, J., Lotterie, J.-A., Demonet, J.-F., Duret, V., Puel, M., Berry, I., Fort, J.-C., Celsis, P., & ADNI (2009). Early diagnosis of Alzheimer’s disease using cortical thickness: impact of cognitive reserve. Brain: A Journal of Neurology, 132, 2036–2047.
Risacher, S. L., Saykin, A. J., West, J. D., Shen, L., Firpi, H. A., & McDonald, B. C. (2009). Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Current Alzheimer Research, 6, 347–361.
Robin, X., Turck, N., Hainard, A., Tiberti, N., Lisacek, F., Sanchez, J. C., & Müller, M. (2011). pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics, 12.
Sabuncu, M. R., Konukoglu, E., & ADNI (2015). Clinical prediction from structural brain MRI scans: a large-scale empirical study. Neuroinformatics, 13, 31–46.
Schwartz, Y., Varoquaux, G., Pallier, C., Pinel, P., Poline, J., & Thirion, B. (2012). Improving accuracy and power with transfer learning using a meta-analytic database. In Proceeding of International Conference on Medical Image Computing and Computer-Assisted Intervention-MICCAI 2012 7512 (pp. 248–255).
Shen, D., & Davatzikos, C. (2002). HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Transactions on Medical Imaging, 21, 1421–1439.
Sled, J. G., Zijdenbos, A. P., & Evans, A. C. (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging, 17, 87–97.
Suk, H., Lee, S. W., Shen, D., & ADNI (2014). Hierarchical feature representation and multimodal fusion with deep learning for AD/MCI diagnosis. NeuroImage, 101, 569–582.
Tibshirani, R. J. (1996). Regression shrinkage and selection via the LASSO. Journal of the Royal Statistical Society, Series B, 58, 267–288.
Wang, Y., Nie, J., Yap, P.-T., Shi, F., Guo, L., Shen, D., 2011. Robust Deformable-Surface-Based Skull-Stripping for Large-Scale Studies. In: Fichtinger, G., Martel, A., Peters, T. (Eds.), Medical Image Computing and Computer-Assisted Intervention. Springer Berlin / Heidelberg, Toronto, Canada, pp. 635–642.
Wee, C. Y., Yap, P. T., Shen, D. G., & ADNI (2013). Prediction of Alzheimer’s disease and mild cognitive impairment using cortical morphological patterns. Human Brain Mapping, 34, 3411–3425.
Westman, E., Muehlboeck, J. S., & Simmons, A. (2012). Combining MRI and CSF measures for classification of Alzheimer’s disease and prediction of mild cognitive impairment conversion. NeuroImage, 62, 229–238.
Westman, E., Aguilar, C., Muehlboeck, J. S., & Simmons, A. (2013). Regional magnetic resonance imaging measures for multivariate analysis in Alzheimer’s disease and mild cognitive impairment. Brain Topography, 26, 9–23.
Wolz, R., Julkunen, V., Koikkalainen, J., Niskanen, E., Zhang, D. P., Rueckert, D., Soininen, H., & Lotjonen, J. (2011). Multi-method analysis of MRI images in early diagnostics of Alzheimer’s disease. PloS One, 6, e25446.
Yang, J., Yan, R., Hauptmann, A.G., (2007). Cross-domain video concept detection using adaptive SVMs. Proceedings of the 15th international conference on Multimedia, 188–197.
Ye, J., Farnum, M., Yang, E., Verbeeck, R., Lobanov, V., Raghavan, N., Novak, G., DiBernardo, A., Narayan, V.A., ADNI, (2012). Sparse learning and stability selection for predicting MCI to AD conversion using baseline ADNI data. BMC Neurology 12, 1471–2377–1412-1446.
Young, J., Modat, M., Cardoso, M. J., Mendelson, A., Cash, D., & Ourselin, S. (2013). Accurate multimodal probabilistic prediction of conversion to Alzheimer’s disease in patients with mild cognitive impairment. NeuroImage: Clinical, 2, 735–745.
Zhang, D., Shen, D., (2011). Semi-supervised multimodal classification of Alzheimer’s disease. Proceeding of IEEE International Symposium on Biomedical Imaging 1628–1631.
Zhang, Y., Brady, M., & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Transactions on Medical Imaging, 20, 45–57.
Zhang, J., Gao, Y., Munsell, B.C., & Shen, D., (2016). Detecting anatomical landmarks for fast alzheimer's disease diagnosis. IEEE Transactions on Medical Imaging. Doi: 10.1109/TMI.2016.2582386.
Zhang, D., Wang, Y., Zhou, L., Yuan, H., Shen, D., & ADNI (2011). Multimodal classification of Alzheimer’s disease and mild cognitive impairment. NeuroImage, 55, 856–867.
Zhang, D., Shen, D., & ADNI (2012). Multi-modal multi-task learning for joint prediction of multiple regression and classification variables in Alzheimer’s disease. NeuroImage, 59, 895–907.
Zhou, J., Liu, J., Narayan, V. A., Ye, J., & ADNI (2013). Modeling disease progression via multi-task learning. NeuroImage, 78, 233–248.
Zhu, X., Huang, Z., Shen, H. T., Cheng, J., & Xu, C. (2012). Dimensionality reduction by mixed kernel canonical correlation analysis. Pattern Recognition, 45, 3003–3016.
Zhu, X., Suk, H., & Shen, D. (2014). A novel matrix-similarity based loss function for joint regression and classification in AD diagnosis. NeuroImage, 100, 91–105.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc., F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., as well as non-profit partners the Alzheimer’s Association and Alzheimer’s Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuron Imaging at the University of California, Los Angeles. This work was supported in part by the National Natural Science Foundation of China (Nos. 61602072, 61422204 and 61473149), the Chongqing Cutting-edge and Applied Foundation Research Program (Nos. cstc2016jcyjA0063, cstc2014jcyjA1316, and cstc2014jcyjA40035), the Scientific and Technological Research Program of Chongqing Municipal Education Commission (Nos. KJ1501014, KJ1401010, and KJ1601003), the NUAA Fundamental Research Funds (No. NE2013105), and NIH grants (AG041721, AG049371, AG042599, AG053867).
Author information
Authors and Affiliations
Consortia
Corresponding authors
Additional information
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu/). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report.
Rights and permissions
About this article
Cite this article
Cheng, B., Liu, M., Shen, D. et al. Multi-Domain Transfer Learning for Early Diagnosis of Alzheimer’s Disease. Neuroinform 15, 115–132 (2017). https://doi.org/10.1007/s12021-016-9318-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12021-016-9318-5