Abstract
Purpose
This study aimed to investigate the effects of randomized, placebo-controlled trials involving the GLP-1 and glucagon receptor dual agonists, mazdutide, and cotadutide, on glycaemic control and body weight changes in individuals with type 2 diabetes mellitus (T2DM), obesity, or both.
Methods
We conducted searches in Medline, PubMed, Scopus, the Cochrane database, and Web of Science up to March 5, 2024. The primary outcomes assessed were changes in HbA1c level and percentage changes in body weight from baseline (CFB).
Results
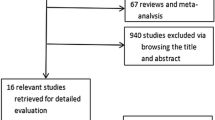
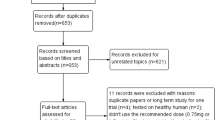
Eleven studies and four unpublished trials were included. The pooled meta-analysis revealed a significant reduction in HbA1c (MD = −0.63%; 95% CI = [−0.82, −0.44]; P < 0.00001), fasting plasma glucose (MD = −1.71 mmol/L; 95% CI = [−2.31, −1.10]; P < 0.00001), and percentage change in body weight (MD = −4.16%; 95% CI = [−5.41, −2.92]; P < 0.00001). Safety analysis revealed no significant change in serious adverse events (OR = 1.03; 95% CI = [0.61, 1.75]; P = 0.91), but there were significantly higher odds of treatment-emergent adverse events (OR = 2.52; 95% CI = [1.92, 3.30]; P < 0.00001) and vomiting (OR = 6.05; 95% CI = [3.52, 10.40]; P < 0.00001).
Conclusion
These results suggest that mazdutide and cotadutide are effective for glycaemic control and weight reduction in individuals with T2DM, obesity, or both.





Similar content being viewed by others
References
H. Sun, P. Saeedi, S. Karuranga, M. Pinkepank, K. Ogurtsova et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119 (2022)
WHO, Obesity. (2024) https://www.who.int/health-topics/obesity#tab=tab_1
X.-F. Pan, L. Wang, A. Pan, Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 9, 373–392 (2021)
E. Ahmad, S. Lim, R. Lamptey, D.R. Webb, M.J. Davies, Type 2 diabetes. Lancet 400, 1803–1820 (2022)
L. Perreault, J.S. Skyler, J. Rosenstock, Novel therapies with precision mechanisms for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 17, 364–377 (2021)
P.Y. Yang, H. Zou, Z. Amso, C. Lee, D. Huang et al. New Generation Oxyntomodulin Peptides with Improved Pharmacokinetic Profiles Exhibit Weight Reducing and Anti-Steatotic Properties in Mice. Bioconjug. Chem. 31, 1167–1176 (2020)
R. Spezani, C.A. Mandarim-de-Lacerda, The current significance and prospects for the use of dual receptor agonism GLP-1/Glucagon. Life Sci. 288, 120188 (2022)
R. Scott, J. Minnion, T. Tan, S.R. Bloom, Oxyntomodulin analogue increases energy expenditure via the glucagon receptor. Peptides 104, 70–77 (2018)
A. Pocai, Action and therapeutic potential of oxyntomodulin. Mol. Metabol. 3, 241–251 (2014)
V.E.R. Parker, T. Hoang, H. Schlichthaar, F.W. Gibb, B. Wenzel et al., Efficacy and safety of cotadutide, a dual glucagon-like peptide-1 and glucagon receptor agonist, in a randomized phase 2a study of patients with type 2 diabetes and chronic kidney disease. Diabetes Obes. Metabol. 24, 1360–1369 (2022)
B. Zhang, Z. Cheng, J. Chen, X. Zhang, D. Liu et al. Efficacy and Safety of Mazdutide in Chinese Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial. Diabetes care 47, 160–168 (2024)
R. Nahra, T. Wang, K.M. Gadde, J. Oscarsson, M. Stumvoll et al. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults With Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care 44, 1433–1442 (2021)
L. Ji, L. Gao, H. Jiang, J. Yang, L. Yu, et al. Safety and efficacy of a GLP-1 and glucagon receptor dual agonist mazdutide (IBI362) 9 mg and 10 mg in Chinese adults with overweight or obesity: A randomised, placebo-controlled, multiple-ascending-dose phase 1b trial. Eclinicalmedicine 54, 101691 (2022)
D. Moher, A. Liberati, J. Tetzlaff, D.G. Altman, P. Grp, Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 8, 336–341 (2010)
J.A.C. Sterne, J. Savovic, M.J. Page, R.G. Elbers, N.S. Blencowe, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Brit. Med. J. 366, l4898 (2019)
G.H. Guyatt, A.D. Oxman, G.E. Vist, R. Kunz, Y. Falck-Ytter et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926 (2008)
C. Xu, S.A.R. Doi, The robust error meta-regression method for dose-response meta-analysis. Int. J. Evid. Based Healthc. 16, 138–144 (2018)
C. Xu, Y. Liu, P.L. Jia, L. Li, T.Z. Liu et al. The methodological quality of dose-response meta-analyses needed substantial improvement: a cross-sectional survey and proposed recommendations. J. Clin. Epidemiol. 107, 1–11 (2019)
S. Durrleman, R. Simon, Flexible regression models with cubic splines. Stat. Med. 8, 551–561 (1989)
V.E.R. Parker, D. Robertson, T. Wang, D.C. Hornigold, M. Petrone et al. Efficacy, Saf ety, and Mechanistic Insights of Cotadutide, a Dual Receptor Glucagon-Like Peptide-1 and Glucagon Agonist. J. Clin. Endocrinol. Metabol. 105, 803–820 (2020)
V.E.R. Parker, D. Robertson, E. Erazo-Tapia, B. Havekes, E. Phielix et al. Cotadutide promotes glycogenolysis in people with overweight or obesity diagnosed with type 2 diabetes. Nat. Metabo. 5, 2148 (2023)
H. Jiang, S. Pang, Y. Zhang, T. Yu, M. Liu et al. A phase 1b randomised controlled trial of a glucagon-like peptide-1 and glucagon receptor dual agonist IBI362 (LY3305677) in Chinese patients with type 2 diabetes. Nat. Commun. 13, 3613 (2022)
L. Ji, H. Jiang, Z. Cheng, W. Qiu, L. Liao et al. A phase 2 randomised controlled trial of mazdutide in Chinese overweight adults or adults with obesity. Nat. Commun. 14, 8289 (2023)
L. Ji, H. Jiang, P. An, H. Deng, M. Liu et al. IBI362 (LY3305677), a weekly-dose GLP-1 and glucagon receptor dual agonist, in Chinese adults with overweight or obesity: A randomised, placebo-controlled, multiple ascending dose phase 1b study. Eclinicalmedicine 39, 101088 (2021)
M. Asano, A. Sekikawa, M. Sugeno, O. Matsuoka, D. Robertson et al. \Safety/tolerability, efficacy and pharmacokinetics of 600-μg cotadutide in Japanese type 2 diabetes patients with a body mass index of 25 kg/m2 or higher: A phase I, randomized, double-blind, placebo-controlled study. Diabetes Obes. Metabol. 25, 2290–2299 (2023)
M. Asano, A. Sekikawa, H. Kim, R.A. Gasser Jr, D. Robertson et al. Pharmacokinetics, safety, tolerability and efficacy of cotadutide, a glucagon-like peptide-1 and glucagon receptor dual agonist, in phase 1 and 2 trials in overweight or obese participants of Asian descent with or without type 2 diabetes. Diabetes Obes. Metabol. 23, 1859–1867 (2021)
P. Ambery, V.E. Parker, M. Stumvoll, M.G. Posch, T. Heise et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet 391, 2607–2618 (2018)
Nct, A Study to Evaluate the Effect of MEDI0382 on Energy Balance in Overweight and Obese Participants With Type 2 Diabetes Mellitus. (2018) https://clinicaltrials.gov/show/NCT03596177
Nct, A Study to Evaluate the Safety and Tolerability of MEDI0382 in Overweight and Obese Participants With Type 2 Diabetes Mellitus. (2018) https://clinicaltrials.gov/show/NCT03745937
Nct, Safety and Tolerability Study of MEDI0382 in Japanese Preobese or Obese Subjects With Type 2 Diabetes. (2018) https://clinicaltrials.gov/show/NCT03645421
M.J. Davies, V.R. Aroda, B.S. Collins, R.A. Gabbay, J. Green et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 65, 1925–1966 (2022)
S.L. Kristensen, R. Rørth, P.S. Jhund, K.F. Docherty, N. Sattar et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 7, 776–785 (2019)
J. Iqbal, H.X. Wu, N. Hu, Y.H. Zhou, L. Li et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus-a systematic review and meta-analysis of randomized control trials. Obes. Rev. 23, e13435 (2022)
J. Li, K. He, J. Ge, C. Li, Z. Jing, Efficacy and safety of the glucagon-like peptide-1 receptor agonist oral semaglutide in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 172, 108656 (2021)
F.K. Knop, V.R. Aroda, R.D. do Vale, T. Holst-Hansen, P.N. Laursen et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 402, 705–719 (2023)
H.A. Dissanayake, N.P. Somasundaram, Polyagonists in Type 2 Diabetes Management. Curr. Diabetes Rep. 24, 1–12 (2024)
Z. Xie, J. Hu, H. Gu, M. Li, J. Chen, Comparison of the efficacy and safety of 10 glucagon-like peptide-1 receptor agonists as add-on to metformin in patients with type 2 diabetes: a systematic review. Front. Endocrinol. 14, 1244432 (2023)
J. Bucheit, J. Ayers, L. Pamulapati, A. Browning, E. Sisson, A Novel Dual Incretin Agent, Tirzepatide (LY3298176), for the Treatment of Type 2 Diabetes Mellitus and Cardiometabolic Health. J. Cardiovasc. Pharmacol. 80, 171–179 (2022)
J.M. Wilson, A. Nikooienejad, D.A. Robins, W.C. Roell, J.S. Riesmeyer et al. The dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes Obes. Metabol. 22, 2451–2459 (2020)
J. Rosenstock, C. Wysham, J.P. Frías, S. Kaneko, C.J. Lee et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 398, 143–155 (2021)
M.B. Sardar, Z.A. Nadeem, M. Babar, Tirzepatide: A novel cardiovascular protective agent in type 2 diabetes mellitus and obesity. Curr. Probl. Cardiol. 49, 102489 (2024)
T. Coskun, S. Urva, W.C. Roell, H. Qu, C. Loghin et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept. Cell Metab. 34, 1234–1247.e1239 (2022)
J. Rosenstock, J. Frias, A.M. Jastreboff, Y. Du, J. Lou et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet 402, 529–544 (2023)
S. Urva, T. Coskun, M.T. Loh, Y. Du, M.K. Thomas et al. LY3437943, a novel triple GIP, GLP-1, and glucagon receptor agonist in people with type 2 diabetes: a phase 1b, multicentre, double-blind, placebo-controlled, randomised, multiple-ascending dose trial. Lancet 400, 1869–1881 (2022)
A.M. Jastreboff, L.M. Kaplan, J.P. Frías, Q. Wu, Y. Du et al. Triple-Hormone-Receptor Agonist Retatrutide for Obesity - A Phase 2 Trial. N. Engl. J. Med. 389, 514–526 (2023)
Y. Chen, A. Mezo, T. Coskun, M. Song, W.C. Roell et al., Novel Dual Glucagon and Glucagon-Like Peptide-1 Receptor Agonist LY3305677 Improves Glucose Control, Reduces Body Weight, and Increases Energy Expenditure in Mice. Diabetes 70, 682 (2021).
S.J. Henderson, A. Konkar, D.C. Hornigold, J.L. Trevaskis, R. Jackson et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes. Metabol. 18, 1176–1190 (2016)
W. Wu, H.-M. Tong, Y.-S. Li, J. Cui, The effect of semaglutide on blood pressure in patients with type-2 diabetes: a systematic review and meta-analysis. Endocrine 83, 571–584 (2024)
L. Xia, T. Shen, W. Dong, F. Su, J. Wang et al. Comparative efficacy and safety of 8 GLP-1RAs in patients with type 2 diabetes: A network meta-analysis. Diabetes Res. Clin. Pract. 177, 108904 (2021)
N. Sattar, M.M.Y. Lee, S.L. Kristensen, K.R.H. Branch, S. Del Prato et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021)
C. Hölscher, Protective properties of GLP-1 and associated peptide hormones in neurodegenerative disorders. Br. J. Pharmacol. 179, 695–714 (2022)
Funding
This work was supported by the National Key R&D Program of China (2021YFC2701700, 2021YFC2701704); National Natural Science Foundation of China (81971433, 82271749, 82201905); the Fundamental Research Funds for the Central University (SCU2023D006); Clinical research Special Fund of Wu Jieping Medical Foundation (320.6750.2023-24-4).
Author information
Authors and Affiliations
Contributions
B.D. and T.R. conceived the study; B.D. and T.R. made the literature search; T.R. and W.L. extracted and analyzed the data; B.D. wrote the draft; T.R., W.L., J.Y. and R.Z. reviewed the article; S.P. and D.M. supervised the study; R.Z. and D.M. are the project managers. All authors approved the final version of the article.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, B., Ruan, T., Lu, W. et al. Safety and efficacy of GLP-1 and glucagon receptor dual agonist for the treatment of type 2 diabetes and obesity: a systematic review and meta-analysis of randomized controlled trials. Endocrine (2024). https://doi.org/10.1007/s12020-024-03857-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-03857-6