Abstract
Purpose
Transient pregnancy-induced Cushing’s syndrome is a rare condition characterized by the manifestation of symptoms solely during pregnancy, which typically resolve spontaneously following delivery or miscarriage. While it has been established that GNAS is associated with adrenal tumors, its specific role in the pathogenesis of pregnancy-induced Cushing’s syndrome remains uncertain.This work aims to examine the association between GNAS mutation and pregnancy-induced Cushing’s syndrome.
Methods
DNA was extracted from patients’ peripheral blood and tumor tissues for whole-exome sequencing (WES) and Sanger sequencing. We used AlphaFold to predict the protein structure of wild-type and mutant GNAS and to make functional predictions, and immunohistochemistry was used to detect disease-associated protein expression. A review and summary of reported cases of transient pregnancy-induced Cushing’s syndrome induced by pregnancy was conducted.
Results
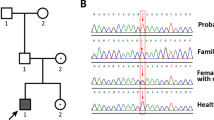
Using WES, we identified a somatic mutation in GNAS (NM_000516, c.C601T, p.R201C) that was predicted to have a deleterious effect using computational methods, such as AlphaFold. Human chorionic gonadotropin (hCG) stimulation tests had weakly positive results, and immunohistochemical staining of adrenal adenoma tissue also revealed positivity for luteinizing hormone/chorionic gonadotropin receptor (LHCGR) and cytochrome P450 family 11 subfamily B member 1 (CYP11B1). We reviewed 15 published cases of transient Cushing’s syndrome induced by pregnancy. Among these cases, immunohistochemical staining of the adrenal gland showed positive LHCGR expression in 3 case reports, similar to our findings.
Conclusion
Transient pregnancy-induced Cushing’s syndrome may be associated with somatic GNAS mutations and altered adrenal pathology due to abnormal activation of LHCGR.


Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Abbreviations
- WES:
-
whole-exome sequencing
- CS:
-
Cushing’s syndrome
- LHCGR:
-
luteinizing hormone/chorionic gonadotropin receptor
- hCG:
-
human chorionic gonadotropin
- CYP11B1:
-
cytochrome P450 family 11 subfamily B member 1
- cAMP:
-
cyclic adenosine monophosphate
- PKA:
-
protein kinase A
- GPCRs:
-
G protein-coupled receptors
- GDP:
-
guanosine diphosphate
- GTP:
-
guanosine triphosphate
References
A. Lacroix, R.A. Feelders, C.A. Stratakis, L.K. Nieman, Cushing’s syndrome. Lancet 386(9996), 913–927 (2015)
N. Younes, M. St-Jean, I. Bourdeau, A. Lacroix, Endogenous Cushing’s syndrome during pregnancy. Rev. Endocr. Metab. Disord. 24(1), 23–38 (2023)
J. Kero, M. Poutanen, F.P. Zhang et al. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J. Clin. Invest 105(5), 633–641 (2000)
U. Plöckinger, M. Chrusciel, M. Doroszko et al. Functional implications of LH/hCG receptors in pregnancy-induced cushing syndrome. J. Endocr. Soc. 1(1), 57–71 (2017)
J. Jumper, R. Evans, A. Pritzel et al. Highly accurate protein structure prediction with AlphaFold. Nature 596(7873), 583–589 (2021)
P.C. Ng, S. Henikoff, SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 31(13), 3812–3814 (2003)
B. Yariv, E. Yariv, A. Kessel et al. Using evolutionary data to make sense of macromolecules with a “face-lifted” ConSurf. Protein Sci. 32(3), e4582 (2023)
I.A. Adzhubei, S. Schmidt, L. Peshkin et al. A method and server for predicting damaging missense mutations. Nat. Methods 7(4), 248–249 (2010)
X. Liu, C. Li, C. Mou, Y. Dong, Y. Tu, dbNSFP v4: a comprehensive database of transcript-specific functional predictions and annotations for human nonsynonymous and splice-site SNVs. Genome Med 12(1), 103 (2020)
D. Szklarczyk, R. Kirsch, M. Koutrouli et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res 51(D1), D638–d646 (2023)
F. Caimari, E. Valassi, P. Garbayo et al. Cushing’s syndrome and pregnancy outcomes: a systematic review of published cases. Endocrine 55(2), 555–563 (2017)
S. Li, C. Yang, J. Fan et al. Pregnancy-induced Cushing’s syndrome with an adrenocortical adenoma overexpressing LH/hCG receptors: a case report. BMC Endocr. Disord. 20(1), 62 (2020)
E. Reschini, G. Giustina, P.G. Crosignani, A. D’Alberton, Spontaneous remission of Cushing syndrome after termination of pregnancy. Obstet. Gynecol. 51(5), 598–602 (1978)
C.E. Andreescu, R.A. Alwani, J. Hofland et al. Adrenal Cushing’s syndrome during pregnancy. Eur. J. Endocrinol. 177(5), K13–k20 (2017)
C. Ruggiero, E. Lalli, Impact of ACTH signaling on transcriptional regulation of steroidogenic genes. Front Endocrinol. (Lausanne) 7, 24 (2016)
D. Goricanec, R. Stehle, P. Egloff et al. Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proc. Natl. Acad. Sci. USA 113(26), E3629–E3638 (2016)
M. Lodish, C.A. Stratakis, A genetic and molecular update on adrenocortical causes of Cushing syndrome. Nat. Rev. Endocrinol. 12(5), 255–262 (2016)
L.S. Weinstein, A. Shenker, P.V. Gejman et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N. Engl. J. Med 325(24), 1688–1695 (1991)
M. Kometani, T. Yoneda, M. Demura et al. Cortisol overproduction results from DNA methylation of CYP11B1 in hypercortisolemia. Sci. Rep. 7(1), 11205 (2017)
J. Rege, J. Hoxie, C.J. Liu et al. Targeted mutational analysis of cortisol-producing adenomas. J. Clin. Endocrinol. Metab. 107(2), e594–e603 (2022)
K. Takedani, M. Yamamoto, S. Tanaka et al. ACTH-independent Cushing’s syndrome due to ectopic endocrinologically functional adrenal tissue caused by a GNAS heterozygous mutation: a rare case of McCune-Albright syndrome accompanied by central amenorrhea and hypothyroidism: a case report and literature review. Front Endocrinol. (Lausanne) 13, 934748 (2022)
M.C. Fragoso, S. Domenice, A.C. Latronico et al. Cushing’s syndrome secondary to adrenocorticotropin-independent macronodular adrenocortical hyperplasia due to activating mutations of GNAS1 gene. J. Clin. Endocrinol. Metab. 88(5), 2147–2151 (2003)
Q. Hu, K.M. Shokat, Disease-causing mutations in the G protein Gαs subvert the roles of GDP and GTP. Cell 173(5), 1254–1264.e1211 (2018)
C. Nwabuobi, S. Arlier, F. Schatz et al. hCG: Biological functions and clinical applications. Int J. Mol. Sci. 18(10), 2037 (2017)
J.E. Pabon, X. Li, Z.M. Lei et al. Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J. Clin. Endocrinol. Metab. 81(6), 2397–2400 (1996)
O. Hatano, A. Takakusu, M. Nomura, K. Morohashi, Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1(7), 663–671 (1996)
A.E. Teo, S. Garg, L.H. Shaikh et al. Pregnancy, primary aldosteronism, and adrenal CTNNB1 mutations. N. Engl. J. Med. 373(15), 1429–1436 (2015)
J. Zhou, E.A.B. Azizan, C.P. Cabrera et al. Somatic mutations of GNA11 and GNAQ in CTNNB1-mutant aldosterone-producing adenomas presenting in puberty, pregnancy or menopause. Nat. Genet 53(9), 1360–1372 (2021)
P. Konstantakou, G. Mastorakos, N. Vrachnis, J.W. Tomlinson, G. Valsamakis, Dysregulation of 11beta-hydroxysteroid dehydrogenases: implications during pregnancy and beyond. J. Matern Fetal Neonatal Med 30(3), 284–293 (2017)
Younes N., St-Jean M., Bourdeau I., Lacroix A. Endogenous Cushing’s syndrome during pregnancy. Rev. Endocr. Metab. Disord. 2022 https://doi.org/10.1007/s11154-022-09731-y.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (82260159,81060220).
Author contributions
Y.L. and J.L. designed the study and participated in data collection. Y.L. performed the systematic review and drafted the manuscript. Z.L. edited and reviewed the manuscript. S.F., L.L., Z.H., H.Y., X.L., Y.Q., J.Z., and D.L. partially conceived the research idea. All authors contributed to the article and approved the submitted version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The studies involving human participants were reviewed and approved by The First Affiliated Hospital of Guangxi Medical University.
Consent to participate
No identifying information of participants was included in the article and verbal consent was obtained from the participants. Written informed consent was not required, as approved by the ethics committee.
Consent to publish
Verbal informed consent was obtained from the participant and this article does not contain any personal details, images or videos.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yufei Li, Jianfan Lin
Supplementary informations
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Lin, J., Fu, S. et al. The mystery of transient pregnancy-induced cushing’s syndrome: a case report and literature review highlighting GNAS somatic mutations and LHCGR overexpression. Endocrine 83, 473–482 (2024). https://doi.org/10.1007/s12020-023-03549-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03549-7