Abstract
Objective
Endocan and vascular endothelial growth factor (VEGF) are markers expressed in various cancer types that are highly vascular, and they have prognostic significance for these cancers. In this study, we aimed to show the expression of endocan and VEGF in pheochromocytoma tumor tissues and to evaluate their correlations with histopathological parameters.
Material and methods
Thirty-eight patients who had been operated for pheochromocytoma were included in the study. As the control group, 28 subjects whose specimens contained normal adrenal medulla tissue were included. The formalin-fixed paraffin-embedded specimens of pheochromocytoma patients were evaluated for Pheochromocytoma of the Adrenal gland Scaled Score (PASS). Sections were then stained for immunohistochemical analysis. The degree of endocan and VEGF positivity was determined by the proportion of stained cells on a negative to strong scale.
Results
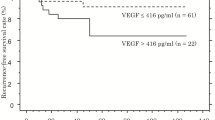
Endocan (p < 0.001) and VEGF (p = 0.004) expressions were found to be significantly higher in the pheochromocytoma group than in the control group. In the pheochromocytoma group, total PASS score (r = 0.714; p < 0.001) and most of the PASS score components were positively correlated with the level of endocan expression. Median Ki-67 index (p = 0.010), total PASS score (p < 0.001), tumor cell spindling (p = 0.048), and nuclear pleomorphism (p = 0.030) were higher in pheochromocytoma with VEGF expression than in those without.
Conclusion
If our findings are supported by studies with a larger sample size, we think that endocan has the potential to be used both as a tumor marker and in predicting malignancy potential in patients with pheochromocytoma, and that the detection of VEGF expression in these tumors is also associated with an increase in malignancy potential.




Similar content being viewed by others
References
L. Amar, J. Bertherat, E. Baudin et al. Genetic testing in pheochromocytoma or functional paraganglioma. J. Clin. Oncol. 23(34), 8812–8818 (2005)
J.W. Lenders, Q.Y. Duh, G. Eisenhofer et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 99(6), 1915–1942 (2014)
P.F. Plouin, P. Fitzgerald, T. Rich, et al. Metastatic pheochromocytoma and paraganglioma: focus on therapeutics. Horm. Metab. Res. 44(5), 390–399 (2012)
L.D. Thompson, Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am. J. Surg. Pathol. 26(5), 551–566 (2002)
A. Kali, K.S. Shetty, Endocan: a novel circulating proteoglycan. Indian J. Pharmacol. 46(6), 579–583 (2014)
M. Delehedde, L. Devenyns, C.A. Maurage, R.R. Vivès, Endocan in cancers: a lesson from a circulating dermatan sulfate proteoglycan. Int. J. Cell Biol. 2013, 705027 (2013)
X. Huang, C. Chen, X. Wang et al. Prognostic value of endocan expression in cancers: evidence from meta-analysis. Onco Targets Ther. 9, 6297–6304 (2016)
C.A. Maurage, E. Adam, J.F. Mineo et al. Endocan expression and localization in human glioblastomas. J. Neuropathol. Exp. Neurol. 68(6), 633–641 (2009)
H. Jiang, X.G. Fu, Y.T. Chen, Serum level of endothelial cell-specific molecule-1 and prognosis of colorectal cancer. Genet. Mol. Res. 14(2), 5519–5526 (2015)
J.H. Kim, M.Y. Park, C.N. Kim et al. Expression of endothelial cell-specific molecule-1 regulated by hypoxia inducible factor-1alpha in human colon carcinoma: impact of ESM-1 on prognosis and its correlation with clinicopathological features. Oncol. Rep. 28(5), 1701–1708 (2012)
K. Ozaki, N. Toshikuni, J. George et al. Serum endocan as a novel prognostic biomarker in patients with hepatocellular carcinoma. J. Cancer 5(3), 221–230 (2014)
K. Hata, Y. Watanabe, H. Nakai, T. Hata, H. Hoshiai, Expression of the vascular endothelial growth factor (VEGF) gene in epithelial ovarian cancer: an approach to anti-VEGF therapy. Anticancer Res. 31(2), 731–737 (2011)
M. Raica, L. Mogoantă, A.M. Cîmpean et al. Immunohistochemical expression of vascular endothelial growth factor (VEGF) in intestinal type gastric carcinoma. Rom. J. Morphol. Embryol. 49(1), 37–42 (2008)
N. Srabovic, Z. Mujagic, J. Mujanovic-Mustedanagic et al. Vascular endothelial growth factor receptor-1 expression in breast cancer and its correlation to vascular endothelial growth factor a. Int. J. Breast Cancer 2013, 746749 (2013)
J. Jacobsen, K. Grankvist, T. Rasmuson, A. Bergh, G. Landberg, B. Ljungberg, Expression of vascular endothelial growth factor protein in human renal cell carcinoma. BJU Int. 93(3), 297–302 (2004)
P. Carmeliet, R.K. Jain, Angiogenesis in cancer and other diseases. Nature 407(6801), 249–257 (2000)
J. Favier, P.F. Plouin, P. Corvol, J.M. Gasc, Angiogenesis and vascular architecture in pheochromocytomas: distinctive traits in malignant tumors. Am. J. Pathol. 161(4), 1235–1246 (2002)
A.A. Ucuzian, A.A. Gassman, A.T. East, H.P. Greisler, Molecular mediators of angiogenesis. J. Burn Care Res. 31(1), 158–175 (2010)
J.C. Varghese, P.F. Hahn, N. Papanicolaou, W.W. Mayo-Smith, J.A. Gaa, M.J. Lee, MR differentiation of phaeochromocytoma from other adrenal lesions based on qualitative analysis of T2 relaxation times. Clin. Radiol. 52(8), 603–606 (1997)
P.P. Parikh, G.A. Rubio, J.C. Farra, J.I. Lew, Nationwide review of hormonally active adrenal tumors highlights high morbidity in pheochromocytoma. J. Surg. Res. 215, 204–210 (2017)
Q. Liu, G. Djuricin, E.D. Staren et al. Tumor angiogenesis in pheochromocytomas and paragangliomas. Surgery 120(6), 938–943 (1996)
H. Ohji, I. Sasagawa, O. Iciyanagi, Y. Suzuki, T. Nakada, Tumour angiogenesis and Ki-67 expression in phaeochromocytoma. BJU Int. 87(4), 381–385 (2001)
K. Salmenkivi, P. Heikkilä, J. Liu, C. Haglund, J. Arola, VEGF in 105 pheochromocytomas: enhanced expression correlates with malignant outcome. APMIS 111(4), 458–464 (2003)
A. Zielke, M. Middeke, S. Hoffmann et al. VEGF-mediated angiogenesis of human pheochromocytomas is associated to malignancy and inhibited by anti-VEGF antibodies in experimental tumors. Surgery 132(6), 1056–1063 (2002)
B.D. Grigoriu, F. Depontieu, A. Scherpereel et al. Endocan expression and relationship with survival in human non-small cell lung cancer. Clin. Cancer Res. 12(15), 4575–4582 (2006)
X. Leroy, S. Aubert, L. Zini et al. Vascular endocan (ESM-1) is markedly overexpressed in clear cell renal cell carcinoma. Histopathology 56(2), 180–187 (2010)
H. Jiang, X.G. Fu, Y.T. Chen, Serum level of endothelial cell-specific molecule-1 and prognosis of colorectal cancer. Genet. Mol. Res. 14(2), 5519–5526 (2015)
S. Moog, F. Castinetti, C. DoCao et al. Recurrence-free survival analysis in locally advanced pheochromocytoma: first appraisal. J. Clin. Endocrinol. Metab. 106(9), 2726–2737 (2021)
Funding
This work was supported by the Scientific Research Fund of Necmettin Erbakan University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The Ethics Committee of the Necmettin Erbakan University (NEU) Meram Medical Faculty approved the study (decision no: 2020/2504 and date: 22.05.2020).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kocabaş, M., Can, M., Karaköse, M. et al. Expression of endocan and vascular endothelial growth factor and their correlation with histopathological prognostic parameters in pheochromocytoma. Endocrine 82, 638–645 (2023). https://doi.org/10.1007/s12020-023-03489-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03489-2