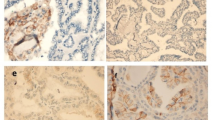
Abstract
Background
The thyroid cancer (THCA) subtype that occurs more frequently is papillary thyroid cancer (PTC). Despite a favorable postoperative outcome, traditional antitumor therapy does not offer ideal results for patients with metastasis, relapse, and radioiodine resistance. Recent studies demonstrated the remarkable effects of immune checkpoint inhibitors on solid tumors, of which the immunoglobulin superfamily member SIGLEC10 and SIGLEC15 act as novel immunotherapy targets for tumors. Nevertheless, their role in PTC prognosis is still indefinite.
Methods
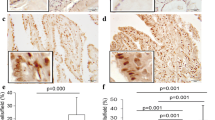
Immunohistochemistry was utilized to examine the expression of SIGLEC10 and SIGLEC15 in 244 PTC tissue specimens. Then the expression correlation between the two was analyzed in normal tissues (NT), tumor cells (TC), and tumor stroma (TS), respectively. Subsequently, the retrospective data on patients with PTC were collected to examine whether the two immunosuppressive SIGLEC family members could affect their prognosis.
Results
We confirmed that TC expressed higher levels of SIGLEC10 than NT. However, SIGLEC10 was down-regulated in TS and predicted poor outcomes. Meanwhile, down-regulation of SIGLEC15 expression was observed in both TC and TS, indicating a favorable prognosis. PTC patients with both SIGLEC10-SIGLEC15+ expression in TC and TS had a significantly higher recurrence risk. The expression of SIGLEC10 in TS and SIGLEC15 in TC or TS was an independent predictor of PFS, and a positive correlation was shown between SIGLEC10 and SIGLEC15 expression in TS.
Conclusions
Therefore, our results indicate that SIGLEC10 and SIGLEC15 may be applied as significant prognostic markers for PTC and attractive targets for THCA immunotherapy.




Similar content being viewed by others
References
H. Sung, J. Ferlay, R.L. Siegel et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). https://doi.org/10.3322/caac.21660
H. Lim, S.S. Devesa, J.A. Sosa, D. Check, C.M. Kitahara, Trends in thyroid cancer incidence and mortality in the united states, 1974–2013. JAMA 317, 1338–1348 (2017). https://doi.org/10.1001/jama.2017.2719
D. Laha, N. Nilubol, M. Boufraqech, New therapies for advanced thyroid cancer. Front Endocrinol (Lausanne) 11, 82 (2020). https://doi.org/10.3389/fendo.2020.00082
W.H. Fridman, F. Pagès, C. Sautès-Fridman, J. Galon, The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012). https://doi.org/10.1038/nrc3245
N.A. Giraldo, R. Sanchez-Salas, J.D. Peske et al. The clinical role of the tme in solid cancer. Br J. Cancer 120, 45–53 (2019). https://doi.org/10.1038/s41416-018-0327-z
S. Maman, I.P. Witz, A history of exploring cancer in context. Nat. Rev. Cancer 18, 359–376 (2018). https://doi.org/10.1038/s41568-018-0006-7
F. Klemm, J.A. Joyce, Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol 25, 198–213 (2015). https://doi.org/10.1016/j.tcb.2014.11.006
G.L. Beatty, W.L. Gladney, Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 21, 687–692 (2015). https://doi.org/10.1158/1078-0432.Ccr-14-1860
S.L. Topalian, C.G. Drake, D.M. Pardoll, Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015). https://doi.org/10.1016/j.ccell.2015.03.001
X. Hou, C. Chen, X. Lan, X. He, Unveiling the molecular features, relevant immune and clinical characteristics of siglec15 in thyroid cancer. Front Immunol 13, 975787 (2022). https://doi.org/10.3389/fimmu.2022.975787
J. Capdevila, L.J. Wirth, T. Ernst et al. Pd-1 blockade in anaplastic thyroid carcinoma. J. Clin. Oncol. 38, 2620–2627 (2020). https://doi.org/10.1200/jco.19.02727
S. Ahn, T.H. Kim, S.W. Kim et al. Comprehensive screening for pd-l1 expression in thyroid cancer. Endocr. Relat. Cancer 24, 97–106 (2017). https://doi.org/10.1530/erc-16-0421
Y. Jiang, X. Zhao, J. Fu, H. Wang, Progress and challenges in precise treatment of tumors with pd-1/pd-l1 blockade. Front Immunol 11, 339 (2020). https://doi.org/10.3389/fimmu.2020.00339
M.S. Macauley, P.R. Crocker, J.C. Paulson, Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol 14, 653–666 (2014). https://doi.org/10.1038/nri3737
A.A. Barkal, R.E. Brewer, M. Markovic et al. Cd24 signalling through macrophage siglec-10 is a target for cancer immunotherapy. Nature 572, 392–396 (2019). https://doi.org/10.1038/s41586-019-1456-0
M.A. Stanczak, S.S. Siddiqui, M.P. Trefny et al. Self-associated molecular patterns mediate cancer immune evasion by engaging siglecs on t cells. J. Clin. Invest. 128, 4912–4923 (2018). https://doi.org/10.1172/jci120612
S. van de Wall, K.C.M. Santegoets, E.J.H. van Houtum, C. Büll, G.J. Adema, Sialoglycans and siglecs can shape the tumor immune microenvironment. Trends Immunol 41, 274–285 (2020). https://doi.org/10.1016/j.it.2020.02.001
N. Xiao, X. Zhu, K. Li et al. Blocking siglec-10(hi) tumor-associated macrophages improves anti-tumor immunity and enhances immunotherapy for hepatocellular carcinoma. Exp. Hematol Oncol. 10, 36 (2021). https://doi.org/10.1186/s40164-021-00230-5
H. Wang, Y. Feng, Y. Zhang et al. Siglec10-an immunosuppressor and negative predictor of survival prognosis in gliomas. Front Genet 13, 873655 (2022). https://doi.org/10.3389/fgene.2022.873655
C. Zhang, J. Zhang, F. Liang et al. Innate immune checkpoint siglec10 in cancers: Mining of comprehensive omics data and validation in patient samples. Front Med. 16, 596–609 (2022). https://doi.org/10.1007/s11684-021-0868-z
B. Li, B. Zhang, X. Wang et al. Expression signature, prognosis value, and immune characteristics of siglec-15 identified by pan-cancer analysis. Oncoimmunology 9, 1807291 (2020). https://doi.org/10.1080/2162402x.2020.1807291
R. Takamiya, K. Ohtsubo, S. Takamatsu, N. Taniguchi, T. Angata, The interaction between siglec-15 and tumor-associated sialyl-tn antigen enhances tgf-β secretion from monocytes/macrophages through the dap12-syk pathway. Glycobiology 23, 178–187 (2013). https://doi.org/10.1093/glycob/cws139
X. Hou, C. Chen, X. He, X. Lan, Siglec-15 silencing inhibits cell proliferation and promotes cell apoptosis by inhibiting stat1/stat3 signaling in anaplastic thyroid carcinoma. Dis Markers 2022, 1606404 (2022). https://doi.org/10.1155/2022/1606404
Z. Tang, C. Li, B. Kang, G. Gao, C. Li, Z. Zhang, Gepia: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45, W98–w102 (2017). https://doi.org/10.1093/nar/gkx247
S. Wen, Y. Luo, W. Wu et al. Identification of lipid metabolism-related genes as prognostic indicators in papillary thyroid cancer. Acta Biochim. Biophys Sin (Shanghai) 53, 1579–1589 (2021). https://doi.org/10.1093/abbs/gmab145
R. Qin, C. Li, X. Wang, Z. Zhong, C. Sun, Identification and validation of an immune-related prognostic signature and key gene in papillary thyroid carcinoma. Cancer Cell Int. 21, 378 (2021). https://doi.org/10.1186/s12935-021-02066-9
V.P. Balachandran, M. Gonen, J.J. Smith, R.P. DeMatteo, Nomograms in oncology: More than meets the eye. Lancet Oncol 16, e173–e180 (2015). https://doi.org/10.1016/s1470-2045(14)71116-7
M.A. Zeiger, E.B. Schneider, Braf v600e mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann. Surg. Oncol. 20, 3–4 (2013). https://doi.org/10.1245/s10434-012-2614-x
J. Munday, S. Kerr, J. Ni et al. Identification, characterization and leucocyte expression of siglec-10, a novel human sialic acid-binding receptor. Biochem. J. 355, 489–497 (2001). https://doi.org/10.1042/0264-6021:3550489
G. Whitney, S. Wang, H. Chang et al. A new siglec family member, siglec-10, is expressed in cells of the immune system and has signaling properties similar to cd33. Eur. J. Biochem. 268, 6083–6096 (2001). https://doi.org/10.1046/j.0014-2956.2001.02543.x
S. Gao, X.Z. Liu, L.Y. Wu et al. Long-term elevated siglec-10 in cerebral spinal fluid heralds better prognosis for patients with aneurysmal subarachnoid hemorrhage. Dis Markers 2022, 5382100 (2022). https://doi.org/10.1155/2022/5382100
T. Angata, Y. Tabuchi, K. Nakamura, M. Nakamura, Siglec-15: An immune system siglec conserved throughout vertebrate evolution. Glycobiology 17, 838–846 (2007). https://doi.org/10.1093/glycob/cwm049
Q.T. Li, Z.Z. Huang, Y.B. Chen et al. Integrative analysis of siglec-15 mrna in human cancers based on data mining. J. Cancer 11, 2453–2464 (2020). https://doi.org/10.7150/jca.38747
M.W.L. Quirino, M.C. Pereira, M.F. Deodato de Souza et al. Immunopositivity for siglec-15 in gastric cancer and its association with clinical and pathological parameters. Eur. J. Histochem. 2021; 65. https://doi.org/10.4081/ejh.2021.3174.
H. Liang, Q. Chen, Z. Hu et al. Siglec15 facilitates the progression of non-small cell lung cancer and is correlated with spinal metastasis. Ann. Transl. Med. 10, 281 (2022). https://doi.org/10.21037/atm-22-764
X. Chen, S. Mo, Y. Zhang et al. Analysis of a novel immune checkpoint, siglec-15, in pancreatic ductal adenocarcinoma. J. Pathol Clin. Res. 8, 268–278 (2022). https://doi.org/10.1002/cjp2.260
J. Wang, J. Sun, L.N. Liu et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 25, 656–666 (2019). https://doi.org/10.1038/s41591-019-0374-x
J.A. Fagin, S.A. Wells Jr., Biologic and clinical perspectives on thyroid cancer. N. Engl. J. Med. 375, 1054–1067 (2016). https://doi.org/10.1056/NEJMra1501993
Q.J. Sheng, W.Y. Tian, X.G. Dou et al. Programmed death 1, ligand 1 and 2 correlated genes and their association with mutation, immune infiltration and clinical outcomes of hepatocellular carcinoma. World J. Gastrointest Oncol. 12, 1255–1271 (2020). https://doi.org/10.4251/wjgo.v12.i11.1255
Acknowledgements
We thank Home for Researchers editorial team (www.home-for-researchers.com) for the language editing service. We are also grateful to Professor Long Chen from the Department of Anesthesiology, Zhejiang Provincial People’s Hospital for providing guidance in the study design.
Author contributions
M.H.G. conceived and designed the study. T.F.J. and L.Q.G. collected, processed, and validated data. W.W. and X.L. analyzed data and prepared figures. T.F.J. and L.Q.G. drafted the manuscript. M.H.G. revised the manuscript. All authors read and approved the final manuscript.
Funding
Our research was supported by the National Natural Science Foundation of China under Grant (Nos. U20A20382).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics
This study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital. Each patient signed an informed consent form.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, T., Wang, W., Ge, L. et al. The expression of two immunosuppressive SIGLEC family molecules in papillary thyroid cancer and their effect on prognosis. Endocrine 82, 590–601 (2023). https://doi.org/10.1007/s12020-023-03452-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03452-1