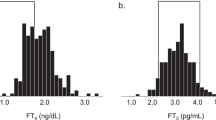
Abstract
Background
The inverse log-linear relationship between Thyroid-stimulating hormone (TSH) and free thyroxine (FT4) is well established and reliably used for evaluation of hypothalamus-pituitary-thyroid (HPT) axis function. However, there are limited data regarding oncologic states in the TSH-FT4 relationship. The purpose of this study was to evaluate thyroid pituitary hypothalamic feedback regulation by the inverse log TSH and FT4 relationship in the cancer patient population at the Ohio State University Comprehensive Cancer Center (OSUCCC-James).
Methods
This retrospective study analyzed the correlation between TSH and FT4 results from 18846 outpatient subjects collected in August 2019-November 2021 at the Department of Family Medicine (OSU Wexner Medical Center), Department of Oncology (OSUCCC-James). Patients with diagnoses related to cancers were included in the oncology group. Patients with diagnoses not related to cancers were included in the non-oncology group. Patients of the Department of Endocrinology, Department of Cardiology, Department of Obstetrics & Gynecology and Department of Hematology were excluded from this study. Time of collection for TSH and FT4 was from 7am to 7 pm. Data were analyzed by morning (7am–12pm) and afternoon (12pm–7pm). Spearman correlation and non-linear fit were used for data analysis. Sex differences were analyzed as well in each group.
Results
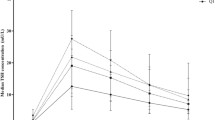
Overall, an inverse correlation was observed between TSH and FT4 in both groups (non-oncology and oncology) regardless of sample collection time and sex differences. Further analysis by linear model in log TSH and FT4 showed a significant inverse fit in males compared with females in the group of oncology, both in the afternoon (p < 0.05). Data were further analyzed by ranges of FT4, as lower or higher (pathophysiology) or within (physiology) the reference interval of FT4. There was no statistical significance between the non-oncology and oncology groups, but relatively good correlation in non-oncology group in either physiologic or pathophysiologic FT4 levels and sample collection time. Interestingly, the best correlation between TSH and FT4 was found in the non-oncology group at pathophysiologic FT4 concentrations (abnormally high). In addition, at pathophysiologic FT4 concentrations (abnormally low), the oncology group demonstrated a significant TSH response in the morning than in the afternoon (p < 0.05).
Conclusions
Though overall the TSH-FT4 curves showed an inverse relationship, there are variations of TSH-FT4 relationship for collection times when considering FT4 in physiologic or pathophysiologic states. The results advance understanding of TSH response, which is beneficial for the interpretation of thyroid disease. We recommend re-evaluation for interpretation of pituitary hypothalamic axis by TSH results when FT4 is abnormally high in oncology patients or low in non-oncology patients, due to poor predictability and the potential for misdiagnosis. A better understanding of the complex nature of the TSH-FT4 relationship may need further study with better defining subclinical states of cancer patients.


Similar content being viewed by others
References
R. Mullur, Y.-Y. Liu, G.A. Brent, Thyroid hormone regulation of metabolism. Physiol. Rev. 94(Apr), 355–382 (2014). https://doi.org/10.1152/physrev.00030.2013
S. Razvi, S. Bhana, S. Mrabeti, Challenges in interpreting thyroid stimulating hormone results in the diagnosis of thyroid dysfunction. J. Thyroid Res. 2019(Sep), 4106816 (2019). https://doi.org/10.1155/2019/4106816
Z. Baloch, P. Carayon, B. Conte-Devolx et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13(Jan), 3–126 (2003). https://doi.org/10.1089/105072503321086962
N. Benhadi, E. Fliers, T.J. Visser, J.B. Reitsma, W.M. Wiersinga, Pilot study on the assessment of the setpoint of the hypothalamus-pituitary-thyroid axis in healthy volunteers. Eur. J. Endocrinol. 162(Feb), 323–329 (2010). https://doi.org/10.1530/EJE-09-0655
C.A. Meier, M.N. Maisey, A. Lowry, J. Müller, M.A. Smith, Interindividual differences in the pituitary-thyroid axis influence the interpretation of thyroid function tests. Clin. Endocrinol. (Oxf) 39(Jul), 101–107 (1993). https://doi.org/10.1111/j.1365-2265.1993.tb01758.x
K.M. Rothacker, S.J. Brown, N.C. Hadlow, R. Wardrop, J.P. Walsh, Reconciling the log-linear and non-log-linear nature of the TSH-Free T4 relationship: intra-individual analysis of a large population. J. Clin. Endocrinol. Metab. 101(Mar), 1151–1158 (2016). https://doi.org/10.1210/jc.2015-4011
N.C. Hadlow, K.M. Rothacker, R. Wardrop, S.J. Brown, E.M. Lim, J.P. Walsh, The relationship between TSH and free T4 in a large population is complex and nonlinear and differs by age and sex. J. Clin. Endocrinol. Metab. 98(Jul), 2936–2943 (2013). https://doi.org/10.1210/jc.2012-4223
D. Strich, G. Karavani, S. Edri, D. Gillis, TSH enhancement of FT4 to FT3 conversion is age dependent. Eur. J. Endocrinol. 175(Jul), 49–54 (2016). https://doi.org/10.1530/EJE-16-0007
M. Wilmar, Wiersinga. Smoking and thyroid. Clin. Endocrinol. (Oxf) 79(Aug), 145–151 (2013). https://doi.org/10.1111/cen.12222
Y. Carter, R.S. Sippel, H. Chen, Hypothyroidism after a cancer diagnosis: etiology, diagnosis, complications, and management. Oncologist. 19(Jan), 34–43 (2014). https://doi.org/10.1634/theoncologist.2013-0237
W.G. Kim, S.-yann Cheng, Thyroid hormone receptors and cancer. Biochim. Biophys. Acta 1830(Jul), 3928–3936 (2013). https://doi.org/10.1016/j.bbagen.2012.04.002
G. Barbesino, Drugs affecting thyroid function. Thyroid 20(Jul), 763–770 (2010). https://doi.org/10.1089/thy.2010.1635
D. Mannavola, P. Coco, G. Vannucchi, R. Bertuelli, M. Carletto, P.G. Casali, P. Beck-Peccoz, L. Fugazzola, A novel tyrosine-kinase selective inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J. Clin. Endocrinol. Metab. 92(Sep), 3531–3534 (2007). https://doi.org/10.1210/jc.2007-0586
H. Miyake, T. Kurahashi, K. Yamanaka, Y. Kondo, M. Muramaki, A. Takenaka, T.-A. Inoue, M. Fujisawa, Abnormalities of thyroid function in Japanese patients with metastatic renal cell carcinoma treated with sorafenib: a prospective evaluation. Urol. Oncol. 28(Sep-Oct), 515–519 (2010). https://doi.org/10.1016/j.urolonc.2009.08.011
C. Giani, P. Fierabracci, R. Bonacci, A. Gigliotti, D. Campani, F. De Negri, D. Cecchetti, E. Martino, A. Pinchera, Relationship between breast cancer and thyroid disease: relevance of autoimmune thyroid disorders in breast malignancy. J. Clin. Endocrinol. Metab. 81(Mar), 990–994 (1996). https://doi.org/10.1210/jcem.81.3.8772562
J.G. Ratcliffe, B.H. Stack, R.W. Burt, W.A. Radcliffe, W.G. Spilg, J. Cuthbert, R.S. Kennedy, Thyroid function in lung cancer. Br. Med. J. 1(Jan), 210–212 (1978). https://doi.org/10.1136/bmj.1.6107.210
E. Krashin, A. Piekiełko-Witkowska, M. Ellis, O. Ashur-Fabian, Thyroid hormones and cancer: a comprehensive review of preclinical and clinical studies. Front. Endocrinol. (Lausanne) 10(Feb), 59 (2019). https://doi.org/10.3389/fendo.2019.00059
J. Gómez-Izquierdo, K.B. Filion, J.-F.ҫois Boivin, L. Azoulay, M. Pollak, O.H.Y. Yu, Subclinical hypothyroidism and the risk of cancer incidence and cancer mortality: a systematic review. BMC Endocr. Disord. 20(Jun), 83 (2020). https://doi.org/10.1186/s12902-020-00566-9
M.V. Deligiorgi, D.T. Trafalis, The clinical relevance of hypothyroidism in patients with solid non-thyroid cancer: a tantalizing conundrum. J. Clin. Med. 11(Jun), 3417 (2022). https://doi.org/10.3390/jcm11123417
O.-P.R. Hamnvik, P.R. Larsen, E. Marqusee, Thyroid dysfunction from antineoplastic agents. J. Natl Cancer Inst. 103(Nov), 1572–1587 (2011). https://doi.org/10.1093/jnci/djr373
Y. Qiu, Y. Hu, Z. Xing, Q. Fu, J. Zhu, A. Su, Birth control pills and risk of hypothyroidism: a cross-sectional study of the National Health and Nutrition Examination Survey, 2007-2012. BMJ Open 11(Jun), e046607 (2021). https://doi.org/10.1136/bmjopen-2020-046607
P.H. Bisschop, A.W. Toorians, E. Endert, W.M. Wiersinga, L.J. Gooren, Eric Fliers. The effects of sex-steroid administration on the pituitary-thyroid axis in transsexuals. Eur. J. Endocrinol. 155(Jul), 11–16 (2006). https://doi.org/10.1530/eje.1.02192
R. Hoermann, A.S. Cheung, M. Milne, M. Grossmann, Hypothalamic-pituitary-thyroid axis set point alterations are associated with body composition in androgen-deprived men. J. Endocr. Soc. 1(May), 874–885 (2017). https://doi.org/10.1210/js.2017-00057
Y. Kajita, M. Ishida, T. Hachiya, T. Miyazaki, M. Yoshimura, H. Ijichi, Y. Ochi, Clinical study on increased serum thyroxine-binding globulin in cancerous state. Endocrinol. Jpn 28(Dec), 785–791 (1981). https://doi.org/10.1507/endocrj1954.28.785
D.P. Rose, T.E. Davis, Plasma thyronine levels in carcinoma of the breast and colon. Arch. Intern. Med. 141(Aug), 1161–1164 (1981)
K. Ikegami, S. Refetoff, E. Van Cauter, T. Yoshimura, Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 15(Oct), 590–600 (2019). https://doi.org/10.1038/s41574-019-0237-z
C. Gallo, V. Fragliasso, B. Donati, F. Torricelli, A. Tameni, S. Piana, A. Ciarrocchi, The bHLH transcription factor DEC1 promotes thyroid cancer aggressiveness by the interplay with NOTCH1. Cell Death Dis. 9(Aug), 871 (2018). https://doi.org/10.1038/s41419-018-0933-y
L.J. DeGroot, Graves’ disease and the manifestations of thyrotoxicosis in Endotext, eds. Feingold K.R. et al. (MDText.com, Inc., 2000), p. 1–77
K. Hartmann, Thyroid disorders in the oncology patient. J. Adv. Pract. Oncol. 6(Mar-Apr), 99–106 (2015). https://doi.org/10.6004/jadpro.2015.6.2.2
T. Nishikawa, S. Yamashita, H. Namba, T. Usa, T. Tominaga, H. Kimura, M. Izumi, S. Nagataki, Interferon-gamma inhibition of human thyrotropin receptor gene expression. J. Clin. Endocrinol. Metab. 77(Oct), 1084–1089 (1993). https://doi.org/10.1210/jcem.77.4.8408457
P.-M. Schumm-Draeger, Sodium/iodide symporter (NIS) and cytokines. Exp. Clin. Endocrinol. Diabetes 109, 32–34 (2001). https://doi.org/10.1055/s-2001-11018
Author information
Authors and Affiliations
Contributions
H.A.: data analysis, interpretation of data; drafted the work. R.W.: interpretation of data; revised the work. J.K.Y.L.: interpretation of data; revised the work. J.J.: interpretation of data; revised it critically for important intellectual content; approved the version to be published. J.L.: design of the work; data analysis, interpretation of data; revised it critically for important intellectual content; approved the version to be published; agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alkhalaileh, H., Wei, R., Lee, J.K.Y. et al. Relationship between TSH and free thyroxine in outpatient cancer patient population. Endocrine 82, 319–325 (2023). https://doi.org/10.1007/s12020-023-03399-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03399-3