Abstract
Purpose
Immune checkpoint inhibitor (ICI) induced type 1 diabetes (T1D) and pituitary dysfunction are life-threatening adverse events, yet there is little clinical data available. We aimed to investigate the clinical characteristics of patients with these adverse events and report their human leukocyte antigen (HLA) profile to determine its relevance.
Methods
This is a single-center prospective study. We enrolled patients with cancers who were administered ICI and diagnosed as ICI induced T1D (ICI-T1D) and pituitary dysfunction (ICI-PD). Clinical data and extracted DNA from blood samples were collected. HLA typing was performed using next-generation sequencing. We compared our results with those previously reported in healthy controls and investigated the correlation between HLA and the occurrence of ICI-T1D and ICI-PD.
Results
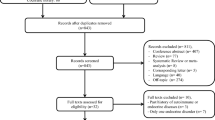
We identified 914 patients treated with ICI in our facility from 1st September, 2017 to 30th June, 2022. Six of these patients developed T1D and 15 developed pituitary dysfunction. The duration from the initiation of ICI treatment to the onset of T1D or pituitary dysfunction averaged 492 ± 196 days and 191 ± 169 days. Among the six patients with T1D, two were positive for anti-GAD antibody. The frequencies of HLA-DR11, -Cw10, -B61, -DRB1*11:01, and -C*03:04 were significantly higher in patients with ICI-T1D than in controls. The frequencies of HLA-DR15 and -DRB*15:02 were significantly higher in patients with ICI-PD than in controls.
Conclusion
This study revealed the clinical characteristics of ICI-T1D and ICI-PD and the association between specific HLAs and these adverse events.



Similar content being viewed by others
References
S.L. Topalian, F.S. Hodi, J.R. Brahmer, S.N. Gettinger, D.C. Smith, D.F. McDermott et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med 366(26), 2443–2454 (2012)
M.A. Postow, M.D. Hellmann, Adverse events associated with immune checkpoint blockade. N. Engl. J. Med 378(12), 1165 (2018)
R. Barroso-Sousa, W.T. Barry, A.C. Garrido-Castro, F.S. Hodi, L. Min, I.E. Krop et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 4(2), 173–182 (2018)
E. Araki, A. Goto, T. Kondo, M. Noda, H. Noto, H. Origasa et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 11(3), 165–223 (2020)
A.M. Stamatouli, Z. Quandt, A.L. Perdigoto, P.L. Clark, H. Kluger, S.A. Weiss et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 67(8), 1471–80. (2018)
S. Yano, K. Ashida, R. Sakamoto, C. Sakaguchi, M. Ogata, K. Maruyama et al. Human leucocyte antigen DR15, a possible predictive marker for immune checkpoint inhibitor-induced secondary adrenal insufficiency. Eur. J. Cancer. 130, 198–203 (2020)
T. Kobayashi, S. Iwama, D. Sugiyama, Y. Yasuda, T. Okuji, M. Ito et al. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J. Immunother. Cancer 9(5), e002493 (2021)
H. Inaba, H. Ariyasu, H. Iwakura, C. Kurimoto, K. Takeshima, S. Morita et al. Distinct clinical features and prognosis between persistent and temporary thyroid dysfunctions by immune-checkpoint inhibitors. Endocr. J. 68(2), 231–241 (2021)
K.E. King, P.M. Ness, H.G. Braine, K.S. Armstrong, Racial differences in the availability of human leukocyte antigen-matched platelets. J. Clin. Apher 11(2), 71–77 (1996)
H. Arima, S. Iwama, H. Inaba, H. Ariyasu, N. Makita, M. Otsuki et al. Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: clinical guidelines of the Japan Endocrine Society. Endocr. J. 66(7), 581–586 (2019)
A. Imagawa, T. Hanafusa, T. Awata, H. Ikegami, Y. Uchigata, H. Osawa et al. Report of the Committee of the Japan Diabetes society on the research of fulminant and acute-onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J. Diabetes Investig 3(6), 536–539 (2012)
N. Ikeda, H. Kojima, M. Nishikawa, K. Hayashi, T. Futagami, T. Tsujino et al. Determination of HLA-A, -C, -B, -DRB1 allele and haplotype frequency in Japanese population based on family study. Tissue Antigens 85(4), 252–259 (2015)
J.M. Wenzlau, K. Juhl, L. Yu, O. Moua, S.A. Sarkar, P. Gottlieb et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc. Natl. Acad. Sci. USA. 104(43), 17040–17045 (2007)
Lo, V. Preiato, S. Salvagni, C. Ricci, A. Ardizzoni, U. Pagotto, C. Pelusi, Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity? Review of the literature. Rev Endocr Metab Disord 22(2), 337–349 (2021)
J.M.K. de Filette, J.J. Pen, L. Decoster, T. Vissers, B. Bravenboer, B.J. Van der Auwera et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur. J. Endocrinol 181(3), 363–374 (2019)
Y. Shi, M. Shen, X. Zheng, Y. Chen, R. Zhao, Y. Gu et al. ICPis-induced autoimmune polyendocrine syndrome type 2: a review of the literature and a protocol for optimal management. J. Clin. Endocrinol. Metab. 105(12), e4208–e4218 (2020)
H. Inaba, Y. Kaido, S. Ito, T. Hirobata, G. Inoue, T. Sugita et al. Human leukocyte antigens and biomarkers in type 1 diabetes mellitus induced by immune-checkpoint inhibitors. Endocrinol. Metab. (Seoul). 37(1), 84–95 (2022)
K. Hamaguchi, A. Kimura, N. Seki, T. Higuchi, S. Yasunaga, M. Takahashi et al. Analysis of tumor necrosis factor-alpha promoter polymorphism in type 1 diabetes: HLA-B and -DRB1 alleles are primarily associated with the disease in Japanese. Tissue Antigens 55(1), 10–16 (2000)
I. Les, M. Martínez, I. Pérez-Francisco, M. Cabero, L. Teijeira, V. Arrazubi et al. Predictive biomarkers for checkpoint inhibitor immune-related adverse events. Cancers (Basel). 15(5), 1629 (2023)
Hasan, O. Ali, F. Berner, D. Bomze, M. Fässler, S. Diem, A. Cozzio et al. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur. J. Cancer 107, 8–14 (2019)
L.C. Cappelli, M.T. Dorak, M.P. Bettinotti, C.O. Bingham, A.A. Shah, Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology (Oxford) 58(3), 476–480 (2019)
Acknowledgements
We thank Ms. Yoshiko Togawa (Tokyo Medical University) for her technical assistance.
Author contributions
The study was designed by H. S. and F. Y.. Data analysis was performed by N. H., who also wrote the manuscript. H. S., K. I., H. I., H. A., H. S., J. S., T. M., and R. S. contributed to the discussion and reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by JSPS KAKENHI (Grant number JP22K07430) and Manda Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Financial interests: H.Suwanai receives personal fees from Nippon Boehringer Ingelheim Co., Ltd. R.S. receives personal fees from Ono Pharmaceutical CO., Ltd, and MSD K.K. N.H., F.Y., K.I., H.I., H.A., H.Sakai., J.S. and T.M. have no financial interests to disclose. All authors have no relevant no non-financial interests to disclose.
Ethical approval
The study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the institutional review board of Tokyo Medical University (Approval No. T2021-0005). The design of this prospective study is in accordance with STROBE guidelines.
Consent to participate
Written informed consents were obtained from all individual patients included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hara, N., Suwanai, H., Yakou, F. et al. Clinical characteristics and human leukocyte antigens in patients with immune checkpoint inhibitor-induced type 1 diabetes and pituitary dysfunction: a single center prospective study. Endocrine 81, 477–483 (2023). https://doi.org/10.1007/s12020-023-03394-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03394-8