Abstract
Purpose
The aim of this study was to investigate the microRNA (miRNA) expression profile in peripheral blood mononuclear cells (PBMC) of thyroid-associated ophthalmopathy (TAO) patients and to explore the molecular mechanisms of MicroRNA-376b (miR-376b) in the pathogenesis of TAO.
Methods
PBMCs from TAO patients and healthy controls were analyzed by miRNA microarray to screen for the significantly differentially expressed miRNAs. The miR-376b expression in PBMCs were confirmed by quantitative real-time polymerase chain reaction (qRT-PCR). The downstream target of miR-376b was screened by online bioinformatics, and detected by qRT-PCR and Western blotting.
Results
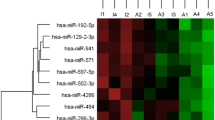
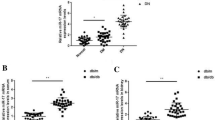
Compared with normal controls, 26 miRNAs were significantly different in PBMCs of TAO patients (14 miRNAs were down-regulated and 12 miRNAs were up-regulated). Among them, miR-376b expression was significantly decreased in PBMCs from TAO patients compared to healthy controls. Spearman correlation analysis revealed that miR-376b expression in PBMCs was significantly negatively correlated with free triiodothyronine (FT3), and positively correlated with thyroid-stimulating hormone (TSH). MiR-376b expression was obviously reduced in 6T-CEM cells after triiodothyronine (T3) stimulation compared to controls. MiR-376b mimics significantly decreased hyaluronan synthase 2 (HAS2) protein expression and the mRNA expression of intercellular cell adhesion molecule-1 (ICAM1) and tumor necrosis factor-α (TNF-α) in 6T-CEM cells, whereas miR-376b inhibitors markedly elevated HAS2 protein expression and gene expression of ICAM1 and TNF-α.
Conclusions
MiR-376b expression in PBMCs was significantly decreased in PBMCs from TAO patients compared with the healthy controls. MiR-376b, regulated by T3, could modulate the expression of HAS2 and inflammatory factors. We speculate that miR-376b may be involved in the pathogenesis of TAO patients by regulating the expression of HAS2 and inflammatory factors.






Similar content being viewed by others
References
A.P. Weetman,, Graves’ disease. N. Engl. J. Med. 343, 1236–1248 (2000). https://doi.org/10.1056/NEJM200010263431707
R. Fernando, T.J. Smith, Slit2 regulates hyaluronan & cytokine synthesis in fibrocytes: potential relevance to thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab. 106, 20 (2021). https://doi.org/10.1210/clinem/dgaa684
Z. Liu, Y. Liu, M. Liu, Q. Gong, A. Shi, X. Li, X. Bai, X. Guan, B. Hao, F. Liu, X. Zhou, H. Yuan, PD-L1 inhibits T cell-induced cytokines and hyaluronan expression via the CD40-CD40L pathway in orbital fibroblasts from patients with thyroid associated ophthalmopathy. Front. Immunol. 13, 849480 (2022). https://doi.org/10.3389/fimmu.2022.849480
W. Yingsung, L. Zhuo, M. Morgelin, M. Yoneda, D. Kida, H. Watanabe, N. Ishiguro, H. Iwata, K. Kimata, Molecular heterogeneity of the SHAP-hyaluronan complex. Isolation and characterization of the complex in synovial fluid from patients with rheumatoid arthritis. J. Biol. Chem. 278, 32710–32718 (2003). https://doi.org/10.1074/jbc.M303658200
K.A. Scheibner, M.A. Lutz, S. Boodoo, M.J. Fenton, J.D. Powell, M.R. Horton, Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 177, 1272–1281 (2006). https://doi.org/10.4049/jimmunol.177.2.1272
L. Zhang, T. Bowen, F. Grennan-Jones, C. Paddon, P. Giles, J. Webber, R. Steadman, M. Ludgate, Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction. J. Biol. Chem. 284, 26447–26455 (2009). https://doi.org/10.1074/jbc.M109.003616
J.M. Cao, N. Wang, S.Y. Hou, X. Qi, W. Xiong, Epigenetics effect on pathogenesis of thyroid-associated ophthalmopathy. Int. J. Ophthalmol. 14, 1441–1448 (2021). https://doi.org/10.18240/ijo.2021.09.22
W.J. Yang, P.F. Ma, S.P. Li et al. MicroRNA-146a contributes to CD4(+) T lymphocyte differentiation in patients with thyroid ophthalmopathy. Am. J. Transl. Res. 9, 1801–1809 (2017)
K. Li, Y. Du, B.L. Jiang, J.F. He, Increased microRNA-155 and decreased microRNA-146a may promote ocular inflammation and proliferation in Graves’ ophthalmopathy. Med. Sci. Monit. 20, 639–643 (2014). https://doi.org/10.12659/MSM.890686
J. Thiel, C. Alter, S. Luppus, A. Eckstein, S. Tan, D. Führer, E. Pastille, A.M. Westendorf, J. Buer, W. Hansen, MicroRNA-183 and microRNA-96 are associated with autoimmune responses by regulating T cell activation. J. Autoimmun. 96, 94–103 (2019). https://doi.org/10.1016/j.jaut.2018.08.010
S.Y. Jang, S.J. Park, M.K. Chae, J.H. Lee, E.J. Lee, J.S. Yoon, Role of microRNA-146a in regulation of fibrosis in orbital fibroblasts from patients with Graves’ orbitopathy. Br. J. Ophthalmol. 102, 407–414 (2018). https://doi.org/10.1136/bjophthalmol-2017-310723
B.D. Tong, M.Y. Xiao, J.X. Zeng, W. Xiong, MiRNA-21 promotes fibrosis in orbital fibroblasts from thyroid-associated ophthalmopathy. Mol. Vis. 21, 324–334 (2015)
J. Tan, B.D. Tong, Y.J. Wu, W. Xiong, MicroRNA-29 mediates TGFbeta1-induced extracellular matrix synthesis by targeting wnt/beta-catenin pathway in human orbital fibroblasts. Int. J. Clin. Exp. Pathol. 7, 7571–7577 (2014)
S.Y. Jang, M.K. Chae, J.H. Lee, E.J. Lee, J.S. Yoon, MicroRNA-27 inhibits adipogenic differentiation in orbital fibroblasts from patients with Graves’ orbitopathy. PLoS One 14, 0221077 (2019). https://doi.org/10.1371/journal.pone.0221077
R. Martínez-Hernández, M. Sampedro-Núñez, A. Serrano-Somavilla, A.M. Ramos-Leví, H. de la Fuente, J.C. Triviño, A. Sanz-García, F. Sánchez-Madrid, M. Marazuela, A MicroRNA signature for evaluation of risk and severity of autoimmune thyroid diseases. J. Clin. Endocrinol. Metab. 103, 1139–1150 (2018). https://doi.org/10.1210/jc.2017-02318
L. Shen, F. Huang, L. Ye, W. Zhu, X. Zhang, S. Wang, W. Wang, G. Ning, Circulating microRNA predicts insensitivity to glucocorticoid therapy in Graves’ ophthalmopathy. Endocrine 49, 445–456 (2015). https://doi.org/10.1007/s12020-014-0487-4
R. Liu, X. Ma, L. Xu, D. Wang, X. Jiang, W. Zhu, B. Cui, G. Ning, D. Lin, S. Wang, Differential microRNA expression in peripheral blood mononuclear cells from Graves’ disease patients. J. Clin. Endocrinol. Metab. 97, E968–E972 (2012). https://doi.org/10.1210/jc.2011-2982
G.B. Bartley, C.A. Gorman, Diagnostic criteria for Graves’ ophthalmopathy. Am. J. Ophthalmol. 119, 792–795 (1995). https://doi.org/10.1016/s0002-9394(14)72787-4
L. Bartalena, G.J. Kahaly, L. Baldeschi, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. (2021). https://doi.org/10.1530/EJE-21-0479
G.B. Bartley, Rundle and his curve. Arch. Ophthalmol. 129, 356 (2011). https://doi.org/10.1001/archophthalmol.2011.29
W.M. Wiersinga. Advances in treatment of active, moderate-to-severe Graves’ ophthalmopathy. Lancet Diabetes Endocrinol. (2017). https://doi.org/10.1016/S2213-8587(16)30046-8
M. Bajénoff, J.G. Egen, L.Y. Koo, J.P. Laugier, F. Brau, N. Glaichenhaus, R.N. Germain, Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989–1001 (2006). https://doi.org/10.1016/j.immuni.2006.10.011
R. Galandrini, N. Albi, G. Tripodi, D. Zarcone, A. Terenzi, A. Moretta, C.E. Grossi, A. Velardi, Antibodies to CD44 trigger effector functions of human T cell clones. J. Immunol. 150, 4225–4235 (1993)
D. Jiang, J. Liang, P.W. Noble, Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 91, 221–264 (2011). https://doi.org/10.1152/physrev.00052.2009
A.G. Gianoukakis, T.A. Jennings, C.S. King, C.E. Sheehan, N. Hoa, P. Heldin, T.J. Smith, Hyaluronan accumulation in thyroid tissue: evidence for contributions from epithelial cells and fibroblasts. Endocrinology 148, 54–62 (2007). https://doi.org/10.1210/en.2006-0736
T.S. Wilkinson, S. Potter-Perigo, C. Tsoi, L.C. Altman, T.N. Wight, Pro- and anti-inflammatory factors cooperate to control hyaluronan synthesis in lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 31, 92–99 (2004). https://doi.org/10.1165/rcmb.2003-0380OC
W. Zhang, T. Liu, T. Li, X. Zhao. Hsa_circRNA_102002 facilitates metastasis of papillary thyroid cancer through regulating miR-488-3p/HAS2 axis. Cancer Gene Ther. (2021). https://doi.org/10.1038/s41417-020-00218-z
Y.L. Sun, S.H. Li, L. Yang, Y. Wang, miR-376b-3p attenuates mitochondrial fission and cardiac hypertrophy by targeting mitochondrial fission factor. Clin. Exp. Pharmacol. Physiol. 45, 779–787 (2018). https://doi.org/10.1111/1440-1681.12938
S. Lu, H. Jiao, J. Xu, Y. Zheng, Y. Sun, H. Chen, Downregulation of IL6 Targeted MiR-376b may contribute to a positive IL6 feedback loop during early liver regeneration in mice. Cell Physiol. Biochem. 37, 233–242 (2015). https://doi.org/10.1159/000430348
G. Korkmaz, C. le Sage, K.A. Tekirdag, R. Agami, D. Gozuacik, miR-376b controls starvation and mTOR inhibition-related autophagy by targeting ATG4C and BECN1. Autophagy 8, 165–176 (2012). https://doi.org/10.4161/auto.8.2.18351
Z. Liu, C. Tang, L. He, D. Yang, J. Cai, J. Zhu, S. Shu, Y. Liu, L. Yin, G. Chen, Y. Liu, D. Zhang, Z. Dong. The negative feedback loop of NF-kappaB/miR-376b/NFKBIZ in septic acute kidney injury. JCI Insight 5 (2020). https://doi.org/10.1172/jci.insight.142272
J. Kovacova, J. Juracek, A. Poprach, J. Kopecky, O. Fiala, M. Svoboda, P. Fabian, L. Radova, P. Brabec, T. Buchler, O. Slaby, MiR-376b-3p is associated with long-term response to sunitinib in metastatic renal cell carcinoma patients. Cancer Genomics Proteomics 16, 353–359 (2019). https://doi.org/10.21873/cgp.20140
X.W. Liu, C.C. Zhang, T. Zhang. MiR-376b-3p functions as a tumor suppressor by targeting KLF15 in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. (2020). https://doi.org/10.26355/eurrev_202009_23033
Acknowledgements
We are grateful to the nurses at the Department of Endocrinology and Metabolism, Tongji Hospital, School of Medicine, Tongji University, for their help in blood collection.
Funding
This study was sponsored by the National Natural Science Foundation of China (Grant No. 81974105, 8227032471, 82200970), Medical Science and Technology Project of Xuhui District, Shanghai (SHXH201735).
Author information
Authors and Affiliations
Contributions
Y.X. and P.F. contributed to the study conception and design. Material preparation was performed by Z.Y., R.L., L.Z., and K.Z. Data collection was performed by R.L., Q.L., and M.X. Analysis were performed by Z.Y. The first draft of the manuscript was written by R.L. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Tongji Hospital, School of Medicine, Tongji University (Approval Number: K-2022-009).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, R., Ye, Z., Liu, Q. et al. MicroRNA-376b is involved in the pathogenesis of thyroid-associated ophthalmopathy by regulating HAS2. Endocrine 82, 87–95 (2023). https://doi.org/10.1007/s12020-023-03382-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03382-y