Abstract
Purpose
The co-occurrence of cyanotic congenital heart disease (CCHD) and PHEO/PGL has been reported, but the role of the hypoxic environment in the pathogenesis of PHEO/PGL remains unclear. Our aim was to compare plasma metanephrine and normetanephrine levels between patients with CCHD and patients with acyanotic congenital heart disease (ACCHD).
Methods
We performed a cross-sectional study in a prospective cohort of 44 patients with congenital heart disease (CHD) (31 (70.5%) females) with a median age of 37.5 (31.0–55.6) years at the time of evaluation. Thirty-two (73%) patients had CCHD and 12 (27%) patients had ACCHD. Morning blood samples for plasma determination of metanephrine and normetanephrine were collected.
Results
Plasma normetanephrine levels were significantly higher in patients with CCHD compared to ACCHD (p = 0.002). Ten (31.3%) patients with CCHD had plasma normetanephrine levels elevated above the reference range, while all ACCHD patients had normal levels. Patients with lower oxygen saturation and higher proBNP had significantly higher normetanephrine levels (ρ = −0.444, p = 0.003 and ρ = 0.449, p = 0.002, respectively). No chromaffin cell tumors were detected.
Conclusion
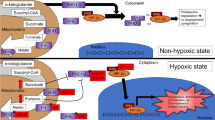
Increased plasma normetanephrine levels in patients with CCHD can be explained by the effect of hypoxia. Future research is needed to better understand the impact of chronic hypoxia in CCHD on increased sympathetic outflow, hyperplastic response of chromaffin tissue, and the role of somatic mutations in CCHD-PHEO/PGL pathogenesis related to hypoxia.

Similar content being viewed by others
References
G.H. Anderson Jr, N. Blakeman, D.H. Streeten, The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J. Hypertens. 12(5), 609–615 (2014). https://doi.org/10.1097/00004872-199405000-00015
J.W. Lenders, Q.Y. Duh, G. Eisenhofer, A.P. Gimenez-Roqueplo, S.K. Grebe et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 99(6), 1915–1942 (2014). https://doi.org/10.1210/jc.2014-1498
F.A. Farrugia, A. Charalampopoulos, Pheochromocytoma. Endocr. Regul. 53(3), 191–212 (2019). https://doi.org/10.2478/enr-2019-0020
G. Eisenhofer, A. Prejbisz, M. Peitzsch, C. Pamporaki, J. Masjkur et al. Biochemical Diagnosis of Chromaffin Cell Tumors in Patients at High and Low Risk of Disease: Plasma versus Urinary Free or Deconjugated O-Methylated Catecholamine Metabolites. Clin. Chem. 64(11), 646–656 (2018). https://doi.org/10.1373/clinchem.2018.291369
S.W. Olson, S. Yoon, T. Baker, L.K. Prince, D. Oliver et al. Longitudinal plasma metanephrines preceding pheochromocytoma diagnosis: a retrospective case-control serum repository study. Eur. J. Endocrinol. 174(3), 289–295 (2016). https://doi.org/10.1530/EJE-15-0651
A.M. Sinclair, C.G. Isles, I. Brown, H. Cameron, G.D. Murray et al. Secondary hypertension in a blood pressure clinic. Arch. Intern Med. 147(7), 1289–1293 (1987)
M. Ariton, C.S. Juan, T.W. AvRuskin, Pheochromocytoma: clinical observations from a Brooklyn tertiary hospital. Endocr. Pr. 6(3), 249–252 (2000). https://doi.org/10.4158/EP.6.3.249
M. Omura, J. Saito, K. Yamaguchi, Y. Kakuta, T. Nishikawa, Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens. Res. 27(3), 193–202 (2004). https://doi.org/10.1291/hypres.27.193
F. Mantero, M. Terzolo, G. Arnaldi, G. Osella, A.M. Masini et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J. Clin. Endocrinol. Metab. 85(2), 637–644 (2000). https://doi.org/10.1210/jcem.85.2.6372
G. Mansmann, J. Lau, E. Balk, M. Rothberg, Y. Miyachi et al. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr. Rev. 25(2), 309–340 (2004). https://doi.org/10.1210/er.2002-0031
L. Fishbein, I. Leshchiner, V. Walter, L. Danilova, A.G. Robertson et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell. 31(2), 181–193 (2017). https://doi.org/10.1016/j.ccell.2017.01.001
A.P. Gimenez-Roqueplo, P.L. Dahia, M. Robledo, An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm. Metab. Res. 44(5), 328–333 (2012). https://doi.org/10.1055/s-0031-1301302
H.Q. Rana, I.R. Rainville, A. Vaidya, Genetic testing in the clinical care of patients with pheochromocytoma and paraganglioma. Curr. Opin. Endocrinol. Diabetes Obes. 21(3), 166–176 (2014). https://doi.org/10.1097/MED.0000000000000059
K. Yamamoto, N. Namba, T. Kubota, T. Usui, K. Takahashi et al. Pheochromocytoma complicated by cyanotic congenital heart disease: a case report. Clin. Pediatr. Endocrinol. 25(2), 59–65 (2016). https://doi.org/10.1297/cpe.25.59
Ogasawara T., Fujii Y., Kakiuchi N., Shiozawa Y., Sakamoto R., et al. Genetic analysis of pheochromocytoma and paraganglioma complicating cyanotic congenital heart disease [published online ahead of print, 2022 Jun 22]. J. Clin. Endocrinol. Metab. 2022;dgac362. https://doi.org/10.1210/clinem/dgac362
M.J. Saldana, L.E. Salem, R. Travezan, High altitude hypoxia and chemodectomas. Hum. Pathol. 4(2), 251–263 (1973). https://doi.org/10.1016/s0046-8177(73)80012-7
D. van der Linde, E.E. Konings, M.A. Slager, M. Witsenburg, W.A. Helbing et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 58(21), 2241–2247 (2011). https://doi.org/10.1016/j.jacc.2011.08.025
Y. Liu, S. Chen, L. Zühlke, G.C. Black, M.K. Choy et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J. Epidemiol. 48(2), 455–463 (2019). https://doi.org/10.1093/ije/dyz009
M.A. Gatzoulis, R. Alonso-Gonzalez, M. Beghetti, Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. Eur. Respir. Rev. 18(113), 154–161 (2009). https://doi.org/10.1183/09059180.00003309
A.R. Opotowsky, L.E. Moko, J. Ginns, M. Rosenbaum, M. Greutmann et al. Pheochromocytoma and paraganglioma in cyanotic congenital heart disease. J. Clin. Endocrinol. Metab. 100(4), 1325–1334 (2015). https://doi.org/10.1210/jc.2014-3863
T. Kita, T. Imamura, H. Date, K. Kitamura, S. Moriguchi et al. Two cases of pheochromocytoma associated with tetralogy of Fallot. Hypertens. Res. 26(5), 433–437 (2003). https://doi.org/10.1291/hypres.26.433
W.H. de Jong, K.S. Graham, J.C. van der Molen, T.P. Links, M.R. Morris et al. Plasma free metanephrine measurement using automated online solid-phase extraction HPLC tandem mass spectrometry. Clin. Chem. 53(9), 1684–1693 (2007). https://doi.org/10.1373/clinchem.2007.087114
P.G. Guyenet, Neural structures that mediate sympathoexcitation during hypoxia. Respir. Physiol. 121(2-3), 147–162 (2000). https://doi.org/10.1016/S0034-5687(00)00125-0
I. Vicario, R. Rigual, A. Obeso, C. Gonzalez, Characterization of the synthesis and release of catecholamine in the rat carotid body in vitro. Am. J. Physiol. Cell Physiol. 278(3), C490–C499 (2000). https://doi.org/10.1152/ajpcell.2000.278.3.C490
S.W. Mifflin, Arterial chemoreceptor input to nucleus tractus solitarius. Am. J. Physiol. 263(2 Pt 2), R368–R375 (1992). https://doi.org/10.1152/ajpregu.1992.263.2.R368
A.S. Hui, J.B. Striet, G. Gudelsky, G.K. Soukhova, E. Gozal et al. Regulation of catecholamines by sustained and intermittent hypoxia in neuroendocrine cells and sympathetic neurons. Hypertension 42(6), 1130–1136 (2003). https://doi.org/10.1161/01.HYP.0000101691.12358.26
F. Fischetti, B. Fabris, M. Zaccaria, A. Biagi, M. Calci et al. Effects of prolonged high-altitude exposure on peripheral adrenergic receptors in young healthy volunteers. Eur. J. Appl. Physiol. 82(5-6), 439–445 (2000). https://doi.org/10.1007/s004210000239
G. Strobel, M. Neureither, P. Bärtsch, Effect of acute mild hypoxia during exercise on plasma free and sulphoconjugated catecholamines. Eur. J. Appl Physiol. Occup. Physiol. 73(1-2), 82–87 (1996). https://doi.org/10.1007/BF00262813
M.S. Balter, K.R. Chapman, M.R. Maleki-Yazdi, F.H. Leenen, A.S. Rebuck, Effects of oxygen withdrawal on catecholamine release in patients on home oxygen therapy. Clin. Sci. (Lond.) 79(2), 155–159 (1990). https://doi.org/10.1042/cs0790155
I. Ponz de Antonio, J. Ruiz Cantador, A.E. González García, J.M. Oliver Ruiz, Á. Sánchez-Recalde, et al., Prevalence of Neuroendocrine Tumors in Patients With Cyanotic Congenital. Heart Dis. Rev. espanola de. cardiologia (Engl. ed.) 70(8), 673–675 (2017). https://doi.org/10.1016/j.rec.2016.09.036
B. Zhao, Y. Zhou, Y. Zhao, Y. Zhao, X. Wu et al. Co-Occurrence of Pheochromocytoma-Paraganglioma and Cyanotic Congenital Heart Disease: A Case Report and Literature Review. Front Endocrinol. (Lausanne) 9, 165 (2018). https://doi.org/10.3389/fendo.2018.00165
D. Amorim-Pires, J. Peixoto, J. Lima, Hypoxia Pathway Mutations in Pheochromocytomas and Paragangliomas. Cytogenet Genome Res. 150(3-4), 227–241 (2016). https://doi.org/10.1159/000457479
A. Vaidya, S.K. Flores, Z.M. Cheng, M. Nicolas, Y. Deng et al. EPAS1 Mutations and Paragangliomas in Cyanotic Congenital Heart Disease. N. Eng. J. Med. 378(13), 1259–1261 (2018). https://doi.org/10.1056/NEJMc1716652
C.M. White, Catecholamines and their blockade in congestive heart failure. Am. J. Health Syst. Pharm. 55(7), 676–682 (1998). https://doi.org/10.1093/ajhp/55.7.676
W.M. Manger, An overview of pheochromocytoma: history, current concepts, vagaries, and diagnostic challenges. Ann. N. Y. Acad. Sci. 1073, 1–20 (2006). https://doi.org/10.1196/annals.1353.001
G. Eisenhofer, G. Rivers, A.L. Rosas, Z. Quezado, W.M. Manger et al. Adverse drug reactions in patients with phaeochromocytoma: incidence, prevention and management. Drug Saf. 30(11), 1031–1062 (2007). https://doi.org/10.2165/00002018-200730110-00004
D.J. Myklejord, Undiagnosed pheochromocytoma: the anesthesiologist nightmare. Clin. Med. Res. 2(1), 59–62 (2004). https://doi.org/10.3121/cmr.2.1.59
A. Prejbisz, J.W. Lenders, G. Eisenhofer, A. Januszewicz, Cardiovascular manifestations of phaeochromocytoma. J. Hypertens. 29(11), 2049–2060 (2011). https://doi.org/10.1097/HJH.0b013e32834a4ce9
D. Weismann, M. Peitzsch, A. Raida, A. Prejbisz, M. Gosk et al. Measurements of plasma metanephrines by immunoassay vs liquid chromatography with tandem mass spectrometry for diagnosis of pheochromocytoma. Eur. J. Endocrinol. 172(3), 251–260 (2015). https://doi.org/10.1530/EJE-14-0730
J.W.M. Lenders, M.N. Kerstens, L. Amar, A. Prejbisz, M. Robledo, D. Taieb et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J. Hypertens. 38(8), 1443–1456 (2020). https://doi.org/10.1097/HJH.0000000000002438
Acknowledgements
We acknowledge all medical staff from the Department of Cardiology, University Medical Centre Ljubljana, Ljubljana, Slovenia for the management of patients with congenital heart disease.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection was performed by Katja Prokšelj, data analysis was performed by Katja Goricar. The first draft of the manuscript was written by Ana Podbregar and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Republic of Slovenia National Medical Ethics Committee with the reference number 0120-134/2019.
Consent to participate
Written informed consent was obtained from each patient.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jensterle, M., Podbregar, A., Janež, A. et al. Comparison of plasma metanephrines in patients with cyanotic and acyanotic congenital heart disease. Endocrine 78, 580–586 (2022). https://doi.org/10.1007/s12020-022-03205-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03205-6