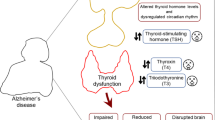
Abstract
Background and objective
Thyroid hormone (TH) disturbances are perceived to contribute to cognitive impairment and dementia. However, there is no consensus on the association between TH levels and Alzheimer Disease (AD). In this study, we aimed to compare serum and cerebrospinal fluid (CSF) TH levels in AD patients to controls by performing a meta-analysis.
Methods
We systematically searched online databases for papers comparing CSF or serum TH levels in AD patients to controls, and performed a meta-analysis on the extracted data.
Results
Out of 1604 records identified, 32 studies were included. No significant difference in serum TSH (standardized mean difference (SMD): −0.03; 95% confidence interval (CI): −0.22–0.16), total T4 (SMD: 0.10; 95% CI: −0.29–0.49), and free T4 (SMD: 0.25; 95% CI: −0.16–0.69) levels were observed. However, there was significantly lower serum total T3 (SMD: −0.56; 95%CI: −0.97 to −0.15) and free T3 (SMD: −0.47; 95%CI: −0.89 to −0.05) levels in AD group compared to controls. Subgroup analyses on studies including only euthyroid patients did not show any significant difference in either of the thyroid hormone levels. Also, no significant difference in CSF total T4 and total T3/total T4 ratios but significantly lower CSF total T3 (SMD: −2.45; 95% CI: −4.89 to −0.02) in AD group were detected.
Conclusion
Serum total and free T3 and CSF total T3 levels are significantly lower in AD patients compared to controls. The temporality of changes in thyroid hormone levels and AD development should be illustrated by further longitudinal studies.





Similar content being viewed by others
Data availability
Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.
References
C.P. Ferri, M. Prince, C. Brayne, H. Brodaty, L. Fratiglioni, M. Ganguli, K. Hall, K. Hasegawa, H. Hendrie, Y. Huang, A. Jorm, C. Mathers, P.R. Menezes, E. Rimmer, M. Scazufca, Global prevalence of dementia: a Delphi consensus study. Lancet. 366(9503), 2112–2117 (2005). https://doi.org/10.1016/s0140-6736(05)67889-0
A. Wimo, B. Winblad, H. Aguero-Torres, E. von Strauss, The magnitude of dementia occurrence in the world. Alzheimer Dis. Assoc. Disord. 17(2), 63–67 (2003). https://doi.org/10.1097/00002093-200304000-00002
Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016 (2019). Lancet Neurol. 18 (1):88–106. https://doi.org/10.1016/s1474-4422(18)30403-4
R. Brookmeyer, E. Johnson, K. Ziegler-Graham, H.M. Arrighi, Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dement.: J. Alzheimer’s Assoc. 3(3), 186–191 (2007). https://doi.org/10.1016/j.jalz.2007.04.381
A. Salehipour, M. Bagheri, M. Sabahi, M. Dolatshahi, D. Boche, Combination therapy in Alzheimer’s disease: is it time? J. Alzheimer's Dis. 87(4), 1433–1449 (2022). https://doi.org/10.3233/jad-215680
A. Serrano-Pozo, M.P. Frosch, E. Masliah, B.T. Hyman, Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 1(1), a006189 (2011). https://doi.org/10.1101/cshperspect.a006189
M.E. Bégin, M.F. Langlois, D. Lorrain, S.C. Cunnane, Thyroid function and cognition during aging. Curr. Gerontol. Geriatr. Res. 2008, 474868 (2008). https://doi.org/10.1155/2008/474868
K. Bavarsad, M. Hosseini, M.A. Hadjzadeh, A. Sahebkar, The effects of thyroid hormones on memory impairment and Alzheimer’s disease. J. Cell. Physiol. (2009) https://doi.org/10.1002/jcp.28198
Y. Hu, Z.C. Wang, Q.H. Guo, W. Cheng, Y.W. Chen, Is thyroid status associated with cognitive impairment in elderly patients in China? BMC Endocr. Disord. 16, 11 (2016). https://doi.org/10.1186/s12902-016-0092-z
A. Akintola, S. Jansen, D. van Bodegom, J. van der Grond, R. Westendorp, A. de Craen, D. Van Heemst, Subclinical hypothyroidism and cognitive function in people over 60 years: a systematic review and meta-analysis. Front. Aging Neurosci. 7 (150), (2015). https://doi.org/10.3389/fnagi.2015.00150
G. Pasqualetti, G. Pagano, G. Rengo, N. Ferrara, F. Monzani, Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 100(11), 4240–4248 (2015). https://doi.org/10.1210/jc.2015-2046
C. Rieben, D. Segna, B.R. da Costa, T-H. Collet, L. Chaker, C.E. Aubert, C. Baumgartner, O.P. Almeida, E. Hogervorst, S. Trompet, K. Masaki, S.P. Mooijaart, J. Gussekloo, R.P. Peeters, D.C. Bauer, D. Aujesky, and N. Rodondi, Thyroid dysfunction and the risk of cognitive decline: a meta-analysis of prospective cohort studies, the journal ofclinical endocrinology & metabolism. 101(12), 4945–4954 (2017). https://doi.org/10.1210/jc.2016-2129
N.A. van Vliet, D. van Heemst, O.P. Almeida, B.O. Åsvold, C.E. Aubert, J.B. Bae, L.E. Barnes, D.C. Bauer, G.J. Blauw, C. Brayne, A.R. Cappola, G. Ceresini, H.C. Comijs, J.F. Dartigues, J.M. Degryse, R.P.F. Dullaart, M.E.A. van Eersel, W.P.J. den Elzen, L. Ferrucci, H.A. Fink, L. Flicker, H.J. Grabe, J.W. Han, C. Helmer, M. Huisman, M.A. Ikram, M. Imaizumi, R.T. de Jongh, J.W. Jukema, K.W. Kim, L.H. Kuller, O.L. Lopez, S.P. Mooijaart, J.H. Moon, E. Moutzouri, M. Nauck, J. Parle, R.P. Peeters, M.H. Samuels, C.O. Schmidt, U. Schminke, P.E. Slagboom, E. Stordal, B. Vaes, H. Völzke, R.G.J. Westendorp, M. Yamada, B.B. Yeap, N. Rodondi, J. Gussekloo, S. Trompet, Association of thyroid dysfunction with cognitive function: an individual participant data analysis. JAMA Intern. Med. 181(11), 1440–1450 (2021). https://doi.org/10.1001/jamainternmed.2021.5078
S. Mohammadi, M. Dolatshahi, F. Rahmani, Shedding light on thyroid hormone disorders and Parkinson disease pathology: mechanisms and risk factors. J. Endocrinol. Investig. 44(1), 1–13 (2021). https://doi.org/10.1007/s40618-020-01314-5
A. Heyman, W.E. Wilkinson, J.A. Stafford, M.J. Helms, A.H. Sigmon, T. Weinberg, Alzheimer’s disease: a study of epidemiological aspects. Ann. Neurol. 15(4), 335–341 (1984). https://doi.org/10.1002/ana.410150406
P.B.S. Figueroa, A.F.F. Ferreira, L.R. Britto, A.P. Doussoulin, A.D.S. Torrão, Association between thyroid function and Alzheimer’s disease: a systematic review. Metab. Brain Dis. 36(7), 1523–1543 (2021). https://doi.org/10.1007/s11011-021-00760-1
D. Moher, A. Liberati, J. Tetzlaff, D.G. Altman, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7), e1000097 (2009). https://doi.org/10.1371/journal.pmed.1000097
A. Stang, Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25(9), 603–605 (2010). https://doi.org/10.1007/s10654-010-9491-z
D. Luo, X. Wan, J. Liu, T. Tong, Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27(6), 1785–1805 (2018). https://doi.org/10.1177/0962280216669183
X. Wan, W. Wang, J. Liu, T. Tong, Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135 (2014). https://doi.org/10.1186/1471-2288-14-135
J.P. Higgins, Cochrane handbook for systematic reviews of interventions version 5.0. 1. The Cochrane Collaboration. (2008) http://www.cochrane-handbook.org
J.P. Higgins, S.G. Thompson, J.J. Deeks, D.G. Altman, Measuring inconsistency in meta-analyses. Bmj 327(7414), 557–560 (2003)
J.M. Chen, C.Q. Huang, M. Ai, L. Kuang, Circadian rhythm of TSH levels in subjects with Alzheimer’s disease (AD). Aging Clin. Exp. Res. 25(2), 153–157 (2013). https://doi.org/10.1007/s40520-013-0025-x
J.M. Gómez Sáez, M. Aguilar Barberá, GH response to GH-releasing factor in dementia and its relation with TSH response to TSH-releasing factor. Recent. Prog. Med. 82(10), 514–516 (1991)
L. Yong-Hong, P. Xiao-Dong, H. Chang-Quan, Y. Bo, L. Qing-Xiu, Hypothalamic-pituitary-thyroid axis in patients with Alzheimer disease (AD).J. Investig. Med. 61(3), 578–581 (2013). https://doi.org/10.2310/JIM.0b013e318280aafb
J.M. Gómez, M. Aguilar, M.A. Navarro, J. Ortolá, J. Soler, Secretion of growth hormone and thyroid-stimulating hormone in patients with dementia. Clin. Investig. 72(7), 489–493 (1994). https://doi.org/10.1007/bf00207475
P. Johansson, E.G. Almqvist, J.O. Johansson, N. Mattsson, O. Hansson, A. Wallin, K. Blennow, H. Zetterberg, J. Svensson, Reduced cerebrospinal fluid level of thyroxine in patients with Alzheimer’s disease. Psychoneuroendocrinology 38(7), 1058–1066 (2013). https://doi.org/10.1016/j.psyneuen.2012.10.012
P. Quinlan, A. Horvath, C. Eckerström, A. Wallin, J. Svensson, Altered thyroid hormone profile in patients with Alzheimer’s disease. Psychoneuroendocrinology 121, 104844 (2020). https://doi.org/10.1016/j.psyneuen.2020.104844
S. Sampaolo, A. Campos-Barros, G. Mazziotti, S. Carlomagno, V. Sannino, G. Amato, C. Carella, G. Di Iorio, Increased cerebrospinal fluid levels of 3,3′,5′-triiodothyronine in patients with Alzheimer’s disease. J. Clin. Endocrinol. Metab. 90(1), 198–202 (2005). https://doi.org/10.1210/jc.2004-1083
G. McKhann, D. Drachman, M. Folstein, R. Katzman, D. Price, E.M. Stadlan, Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7), 939–944 (1984). https://doi.org/10.1212/wnl.34.7.939
L.A. van Osch, E. Hogervorst, M. Combrinck, A.D. Smith, Low thyroid-stimulating hormone as an independent risk factor for Alzheimer disease. Neurology 62(11), 1967–1971 (2004). https://doi.org/10.1212/01.wnl.0000128134.84230.9f
R. Agarwal, S. Kushwaha, N. Chhillar, A. Kumar, D.K. Dubey, C.B. Tripathi, A cross-sectional study on thyroid status in North Indian elderly outpatients with dementia. Ann. Indian Acad. Neurol. 16(3), 333–337 (2013). https://doi.org/10.4103/0972-2327.116916
T.H. Lampe, S.R. Plymate, S.C. Risse, H. Kopeikin, L. Cubberley, M.A. Raskind, TSH responses to two TRH doses in men with Alzheimer’s disease. Psychoneuroendocrinology 13(3), 245–254 (1988). https://doi.org/10.1016/0306-4530(88)90022-4
S.E. Molchan, B.A. Lawlor, J.L. Hill, A.M. Mellow, C.L. Davis, R. Martinez, T. Sunderland, The TRH stimulation test in Alzheimer’s disease and major depression: relationship to clinical and CSF measures. Biol. Psychiatry 30(6), 567–576 (1991). https://doi.org/10.1016/0006-3223(91)90026-i
N. Kimura, T. Kumamoto, H. Masuda, T. Hanaoka, Y. Hazama, T. Okazaki, R. Arakawa, Relationship between thyroid hormone levels and regional cerebral blood flow in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 25(2), 138–143 (2011). https://doi.org/10.1097/WAD.0b013e3181f9aff2
S. Nomoto, R. Kinno, H. Ochiai, S. Kubota, Y. Mori, A. Futamura, A. Sugimoto, T. Kuroda, S. Yano, H. Murakami, T. Shirasawa, T. Yoshimoto, A. Minoura, A. Kokaze, K. Ono, The relationship between thyroid function and cerebral blood flow in mild cognitive impairment and Alzheimer’s disease. PLOS One 14(4), e0214676 (2019). https://doi.org/10.1371/journal.pone.0214676
S. Barez-Lopez, A. Guadano-Ferraz, Thyroid hormone availability and action during brain development in rodents. Front. Cell. Neurosci. 11, 240 (2017). https://doi.org/10.3389/fncel.2017.00240
B. Belandia, M.J. Latasa, A. Villa, A. Pascual, Thyroid hormone negatively regulates the transcriptional activity of the beta-amyloid precursor protein gene. J. Biol. Chem. 273(46), 30366–30371 (1998). https://doi.org/10.1074/jbc.273.46.30366
A. Montero-Pedrazuela, C. Venero, R. Lavado-Autric, I. Fernandez-Lamo, J.M. Garcia-Verdugo, J. Bernal, A. Guadano-Ferraz, Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol. Psychiatry 11(4), 361–371 (2006). https://doi.org/10.1038/sj.mp.4001802
C.C. Thompson, G.B. Potter, Thyroid hormone action in neural development. Cereb. Cortex 10(10), 939–945 (2000). https://doi.org/10.1093/cercor/10.10.939
H. Vara, B. Martinez, A. Santos, A. Colino, Thyroid hormone regulates neurotransmitter release in neonatal rat hippocampus. Neuroscience 110(1), 19–28 (2002). https://doi.org/10.1016/s0306-4522(01)00541-3
A. Chaalal, R. Poirier, D. Blum, S. Laroche, V. Enderlin, Thyroid hormone supplementation restores spatial memory, hippocampal markers of neuroinflammation, plasticity-related signaling molecules, and β-amyloid peptide load in hypothyroid rats. Mol. Neurobiol. 56(1), 722–735 (2019). https://doi.org/10.1007/s12035-018-1111-z
L.X. Li, T. Yang, L. Guo, D.Y. Wang, C.H. Tang, Q. Li, H.M. Yang, J. Zhu, L.L. Zhang, Serum tau levels are increased in patients with hyperthyroidism. Neurosci. Lett. 729, 135003 (2020). https://doi.org/10.1016/j.neulet.2020.135003
L. Goumidi, F. Flamant, C. Lendon, D. Galimberti, F. Pasquier, E. Scarpini, D. Hannequin, D. Campion, P. Amouyel, J.C. Lambert, A. Meirhaeghe, Study of thyroid hormone receptor alpha gene polymorphisms on Alzheimer’s disease. Neurobiol. Aging 32(4), 624–630 (2011). https://doi.org/10.1016/j.neurobiolaging.2009.04.007
P. Quinlan, A. Horvath, A. Wallin, J. Svensson, Low serum concentration of free triiodothyronine (FT3) is associated with increased risk of Alzheimer’s disease. Psychoneuroendocrinology 99, 112–119 (2019). https://doi.org/10.1016/j.psyneuen.2018.09.002
E.L. Constant, A.G. de Volder, A. Ivanoiu, A. Bol, D. Labar, A. Seghers, G. Cosnard, J. Melin, C. Daumerie, Cerebral blood flow and glucose metabolism in hypothyroidism: a positron emission tomography study. J. Clin. Endocrinol. Metab. 86(8), 3864–3870 (2001). https://doi.org/10.1210/jcem.86.8.7749
P. Quinlan, A. Horvath, C. Eckerström, A. Wallin, J. Svensson, Higher thyroid function is associated with accelerated hippocampal volume loss in Alzheimer’s disease. Psychoneuroendocrinology 139, 105710 (2022). https://doi.org/10.1016/j.psyneuen.2022.105710
M. Goto, N. Kimura, E. Matsubara, Association of serum thyroid hormone levels with positron emission tomography imaging in non-demented older adults. Psychogeriatrics 22(3), 373–381 (2022). https://doi.org/10.1111/psyg.12825
A. Accorroni, F.S. Giorgi, R. Donzelli, L. Lorenzini, C. Prontera, A. Saba, A. Vergallo, G. Tognoni, G. Siciliano, F. Baldacci, U. Bonuccelli, A. Clerico, R. Zucchi, Thyroid hormone levels in the cerebrospinal fluid correlate with disease severity in euthyroid patients with Alzheimer’s disease. Endocrine 55(3), 981–984 (2017). https://doi.org/10.1007/s12020-016-0897-6
E. Fliers, A.C. Bianco, L. Langouche, A. Boelen, Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 3(10), 816–825 (2015). https://doi.org/10.1016/s2213-8587(15)00225-9
G. Pasqualetti, V. Calsolaro, S. Bernardini, G. Linsalata, R. Bigazzi, N. Caraccio, F. Monzani, Degree of peripheral thyroxin deiodination, frailty, and long-term survival in hospitalized older patients. J. Clin. Endocrinol. Metab. 103(5), 1867–1876 (2018). https://doi.org/10.1210/jc.2017-02149
J. Gussekloo, E. van Exel, A.J. de Craen, A.E. Meinders, M. Frölich, R.G. Westendorp, Thyroid status, disability and cognitive function, and survival in old age. JAMA 292(21), 2591–2599 (2004). https://doi.org/10.1001/jama.292.21.2591
J.D. Davis, A. Podolanczuk, J.E. Donahue, E. Stopa, J.V. Hennessey, L.G. Luo, Y.P. Lim, R.A. Stern, Thyroid hormone levels in the prefrontal cortex of post-mortem brains of Alzheimer’s disease patients. Curr. Aging Sci. 1(3), 175–181 (2008). https://doi.org/10.2174/1874609810801030175
B. Đapić, E. Schernhammer, H. Haslacher, E. Stögmann, J. Lehrner, No effect of thyroid hormones on 5-year mortality in patients with subjective cognitive decline, mild cognitive disorder, and Alzheimer’s disease. J. Neuroendocrinol. 34(4), e13107 (2022). https://doi.org/10.1111/jne.13107
A. Chiaravalloti, F. Ursini, A. Fiorentini, G. Barbagallo, A. Martorana, G. Koch, M. Tavolozza, O. Schillaci, Functional correlates of TSH, fT3 and fT4 in Alzheimer disease: a F-18 FDG PET/CT study. Sci. Rep. 7(1), 6220 (2017). https://doi.org/10.1038/s41598-017-06138-7
E. Marouli, L. Yusuf, A.D. Kjaergaard, R. Omar, A. Kuś, O. Babajide, R. Sterenborg, B.O. Åsvold, S. Burgess, C. Ellervik, A. Teumer, M. Medici, P. Deloukas, Thyroid function and the risk of Alzheimer’s disease: a Mendelian randomization study. Thyroid 31(12), 1794–1799 (2021). https://doi.org/10.1089/thy.2021.0321
G.H. Li, C.L. Cheung, E.Y. Cheung, W.C. Chan, K.C. Tan, Genetically determined TSH level within reference range is inversely associated with Alzheimer disease. J. Clin. Endocrinol. Metab. 106(12), e5064–e5074 (2021). https://doi.org/10.1210/clinem/dgab527
N. Zhang, H.J. Du, J.H. Wang, Y. Cheng, A pilot study on the relationship between thyroid status and neuropsychiatric symptoms in patients with Alzheimer disease. Chin. Med J. 125(18), 3211–3216 (2012)
R. Agarwal, N. Chhillar, S. Kushwaha, N.K. Singh, C.B. Tripathi, Role of vitamin B(12), folate, and thyroid stimulating hormone in dementia: a hospital-based study in north Indian population. Ann. Indian Acad. Neurol. 13(4), 257–262 (2010). https://doi.org/10.4103/0972-2327.74193
Y. Hu, Z.-C. Wang, Q.-H. Guo, W. Cheng, Y.-W. Chen, Is thyroid status associated with cognitive impairment in elderly patients in China? BMC Endocr. Disord. 16(1), 11 (2016). https://doi.org/10.1186/s12902-016-0092-z
D.R. Thomas, R. Hailwood, B. Harris, P.A. Williams, M.F. Scanlon, R. John, Thyroid status in senile dementia of the Alzheimer type (SDAT). Acta Psychiatr. Scand. 76(2), 158–163 (1987). https://doi.org/10.1111/j.1600-0447.1987.tb02879.x
J.E. Christie, L.J. Whalley, J. Bennie, H. Dick, I.M. Blackburn, D.H. Blackwood, G. Fink, Characteristic plasma hormone changes in Alzheimer’s disease. Br. J. Psychiatry 150, 674–681 (1987). https://doi.org/10.1192/bjp.150.5.674
T. Zhao, B.M. Chen, X.M. Zhao, Z.Y. Shan, Subclinical hypothyroidism and depression: a meta-analysis. Transl. Psychiatry 8(1), 239 (2018). https://doi.org/10.1038/s41398-018-0283-7
Y. Zhou, Y. Ma, Q. Wu, Q. Wang, W.F.Z. Yang, Y. Wang, D. Yang, Y. Luo, K. Tang, T. Liu, D. Wang, Comparison of thyroid hormone levels between patients with major depressive disorder and healthy individuals in China. Front. Psychiatry 12 (1716) (2021). https://doi.org/10.3389/fpsyt.2021.750749
M. Albert, M. Jenike, R. Nixon, K. Nobel, Thyrotropin response to thyrotropin-releasing hormone in patients with dementia of the Alzheimer type. Biol. Psychiatry 33(4), 267–271 (1993). https://doi.org/10.1016/0006-3223(93)90293-M
Y.S. Chang, Y.H. Wu, C.J. Wang, S.H. Tang, H.L. Chen, Higher levels of thyroxine may predict a favorable response to donepezil treatment in patients with Alzheimer disease: a prospective, case–control study. BMC Neurosci. 19(1), 36 (2018). https://doi.org/10.1186/s12868-018-0436-x
L.G. Forssell, R. Eklöf, B. Winblad, L. Forssell, Early stages of late onset Alzheimer’s disease. Acta Neurol. Scand. 79(S121), 27–42 (1989). https://doi.org/10.1111/j.1600-0404.1989.tb04875.x
M. Franceschi, L. Perego, L. Ferini-Strambi, S. Smirne, N. Canal, Neuroendocrinological function in Alzheimer’s disease. Neuroendocrinology 48(4), 367–370 (1988). https://doi.org/10.1159/000125036
E. Kapaki, I. Ilias, G.P. Paraskevas, I. Theotoka, I. Christakopoulou, Thyroid function in patients with Alzheimer’s diseasetreated with cholinesterase inhibitors. Acta Neurobiol. Exp 63(4), 389–392 (2003).
N. Ulusu, G. Yilmaz, Z. Erbayraktar, A. Evlice, M. Genc, S. Aras, A. Avci, G. Yener, A comparative study on thyroid function in Alzheimer’s disease: results from a Turkish multi-centre study. J. Neurol. Sci. 32, 335–347 (2015)
C.A. Peabody, J.E. Thornton, J.R. Tinklenberg, Progressive dementia associated with thyroid disease. J. Clin. Psychiatry 47(2), 100 (1986)
E.R. Sarhat, Altered serum marker of thyroid profile and antioxidant enzymes in individuals Alzheimer’s disease. Int. Res. J. Pharm. 10(1), 56–60 (2019).
T. Sunderland, P.N. Tariot, E.A. Mueller, P.A. Newhouse, D.L. Murphy, R.M. Cohen, TRH stimulation test in dementia of the Alzheimer type and elderly controls. Psychiatry Res. 16(4), 269–275 (1985). https://doi.org/10.1016/0165-1781(85)90118-0
S. Annerbo, L.-O. Wahlund, J. Lökk, The significance of thyroid-stimulating hormone and homocysteine in the development of Alzheimer’s disease in mild cognitive impairment: a 6-year follow-up study. Am. J. Alzheimer’s Dis. Other Dement.® 21(3), 182–188 (2006). https://doi.org/10.1177/1533317506289282
S. Annerbo, M. Kivipelto, J. Lokk, A prospective study on the development of Alzheimer’s disease with regard to thyroid-stimulating hormone and homocysteine. Dement. Geriatr. Cogn. Disord. 28(3), 275–280 (2009). https://doi.org/10.1159/000242439
R.M. Ranzola, Y.R. Rodríguez, J.J. Cuesta, A.P. Truffin, J.C. Llano, Myeloperoxidase activity, lipid profile and thyroid function in patients who suffer from Alzheimer’s disease. Rev. Cubana Investig. Bioméd. 38 (1), (2019)
Acknowledgements
We are thankful to the authors of the reviewed/included studies in this systematic review who contributed by sharing the relevant data on request.
Author information
Authors and Affiliations
Contributions
M.D., Am.S., A.F., and A.T. contributed to conception and design of the study, M.D., and Ar.S. contributed to data acquisition and analysis, M.D., Ar.S., H.S.M. contributed to drafting a significant portion of the manuscript and figures, A.T., A.F., and V.A. supervised this study, M.D., Ar.S., Am.S., H.S.M., V.A., A.T., A.F., contributed to revising the manuscript and confirmed the final version of the manucript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study was done in accorance with ethics guidelines of Helsinki. The protocol for this study was registered in the Prospective Register of Systematic Reviews (PROSPERO) website (https://www.crd.york.ac.uk/prospero/) with the following ID: CRD42020078556. It was reviewed and approved by the ethics committee of Tehran University of Medical Sciences and designated the followoing ethics code: IR.TUMS.VCR.REC.1398.559.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dolatshahi, M., Salehipour, A., Saghazadeh, A. et al. Thyroid hormone levels in Alzheimer disease: a systematic review and meta-analysis. Endocrine 79, 252–272 (2023). https://doi.org/10.1007/s12020-022-03190-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03190-w