Abstract
Purpose
This study aimed to analyze the clinical course of patients with differentiated thyroid cancer (DTC) who were treated by lenvatinib and investigate the specific criteria for the initiation of lenvatinib in lung metastasis.
Methods
A total of 111 patients with DTC treated by lenvatinib were included in the study. Patients were divided into two groups based on the target lesion for the initiation of lenvatinib: lung metastasis group and other metastases group.
Results
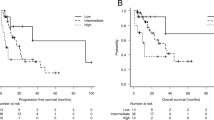
In the univariate analysis, the tumor size for the lung metastasis (p = 0.002) and the factor of lung metastasis group (p < 0.001) were significantly associated with overall survival (OS). Multivariate analysis revealed that the factor of lung metastasis group [hazard ratio, 0.408; 95% confidence interval (CI), 0.206–0.810; p = 0.010] was the only independent prognostic factor of OS. Of the 53 patients in the lung metastasis group, 12 (23%) had lung metastasis-related finding such as pleural effusion (n = 12), hemoptysis (n = 2), and dyspnea (n = 1) at the initiation of lenvatinib treatment. The median OS in patients with or without lung metastasis-related findings were 41.0 [95% CI, 10.4–not available (NA)] months and 62.9 (95% CI, 53.0–NA) months, respectively (p = 0.022).
Conclusion
Patients with lung metastasis-related finding at the initiation of lenvatinib treatment had a poorer prognosis among the lung metastasis group. It is important to consider not only the tumor size but also the presence of lung metastasis-related findings when initiating lenvatinib treatment for DTC patients with lung metastasis.



Similar content being viewed by others
References
R.L. Siegel, K.D. Miller, H.E. Fuchs, A. Jemal, Cancer statistics, 2021. CA Cancer J. Clin. 71, 7–33 (2021)
U. Megwalu, P.K. Moon, Thyroid cancer incidence and mortality trends in the United States: 2000–2018. Thyroid 32, 560–570 (2022)
M.E. Cabanillas, M.A. Habra, Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat. Rev. 42, 47–55 (2016)
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov, F. Pacini, G.W. Randolph, A.M. Sawka, M. Schlumberger, K.G. Schuff, S.I. Sherman, J.A. Sosa, D.L. Steward, R.M. Tuttle, L. Wartofsky, 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26, 1–133 (2016)
M. Schlumberger, M. Tahara, L.J. Wirth, B. Robinson, M.S. Brose, R. Elisei, M.A. Habra, K. Newbold, M.H. Shah, A.O. Hoff, A.G. Gianoukakis, N. Kiyota, M.H. Taylor, S.B. Kim, M.K. Krzyzanowska, C.E. Dutcus, B. de las Heras, J. Zhu, S.I. Sherman, Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 372, 621–630 (2015)
M. Tahara, N. Kiyota, A.O. Hoff, C. Badiu, T.K. Owonikoko, C.E. Dutcus, T. Suzuki, M. Ren, L.J. Wirth, Impact of lung metastases on overall survival in the phase 3 SELECT study of lenvatinib in patients with radioiodine-refractory differentiated thyroid cancer. Eur. J. Cancer 147, 51–57 (2021)
N. Kiyota, M. Tahara, B. Robinson, M. Schlumberger, S.I. Sherman, S. Leboulleux, E.K. Lee, T. Suzuki, M. Ren, K. Fushimi, L.J. Wirth, Impact of baseline tumor burden on overall survival in patients with radioiodine-refractory differentiated thyroid cancer treated with lenvatinib in the SELECT global phase 3 trial. Cancer 128, 2281–2287 (2022)
M.H. Taylor, S. Takahashi, J. Capdevila, M. Tahara, S. Leboulleux, N. Kiyota, C.E. Dutcus, R. Xie, B. Robinson, S. Sherman, M.A. Habra, R. Elisei, L.J. Wirth, Correlation of performance status and neutrophil-lymphocyte ratio with efficacy in radioiodine-refractory differentiated thyroid cancer treated with lenvatinib. Thyroid 31, 1226–1234 (2021)
N. Kiyota, M. Schlumberger, K. Muro, Y. Ando, S. Takahashi, Y. Kawai, L. Wirth, B. Robinson, S. Sherman, T. Suzuki, K. Fujino, A. Gupta, S. Hayato, M. Tahara, Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci. 106, 1714–1721 (2015)
H. Iwasaki, S. Toda, D. Murayama, A. Matsui, Analysis of disease progression and prognosis in differentiated thyroid cancer with pulmonary metastases: a retrospective study. Int. J. Surg. Oncol. 5, e104 (2020)
H. Yamazaki, H. Iwasaki, H. Takasaki, N. Suganuma, R. Sakai, K. Masudo, H. Nakayama, Y. Rino, M. Masuda, Efficacy and tolerability of initial low-dose lenvatinib to treat differentiated thyroid cancer. Medicine 98, e14774 (2019)
E.A. Eisenhauer, P. Therasse, J. Bogaerts, L.H. Schwartz, D. Sargent, R. Ford, J. Dancey, S. Arbuck, S. Gwyther, M. Mooney, L. Rubinstein, L. Shankar, L. Dodd, R. Kaplan, D. Lacombe, J. Verweij, New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009)
Y. Kanda, Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 48, 452–458 (2013)
A.G. Gianoukakis, C.E. Dutcus, N. Batty, M. Guo, M. Baig, Prolonged duration of response in lenvatinib responders with thyroid cancer. Endocr. Relat. Cancer 25, 699–704 (2018)
M.S. Brose, F.P. Worden, K.L. Newbold, M. Guo, A. Hurria, Effect of age on the efficacy and safety of lenvatinib in radioiodine-refractory differentiated thyroid cancer in the phase III SELECT trial. J. Clin. Oncol. 35, 2692–2699 (2017)
D. Wu, C.J. Gomes Lima, S.L. Moreau, K. Kulkarni, A. Zeymo, K.D. Burman, L. Wartofsky, D. Van Nostrand, Improved survival after multimodal approach with 131 I treatment in patients with bone metastases secondary to differentiated thyroid cancer. Thyroid 29, 971–978 (2019)
Y. Saito, K. Sugino, H. Takami, K. Matsuzu, T. Uruno, K. Ohkuwa, W. Kitagawa, M. Nagahama, H. Kawakubo, K. Ito, Y. Kitagawa, Clinical status and treatment of liver metastasis of differentiated thyroid cancer using tyrosine kinase inhibitors. World J. Surg. 42, 3632–3637 (2018)
F. Saito, T. Uruno, H. Shibuya, W. Kitagawa, M. Nagahama, K. Sugino, K. Ito, Prognosis after brain metastasis from differentiated thyroid carcinoma. World J. Surg. 40, 574–581 (2016)
D.T. Broome, G.B. Gadre, E. Fayazzadeh, J.F. Bena, C. Nasr, Pleural effusion as a novel prognostic factor in metastatic thyroid carcinoma. Endocr. Connect 9, 812–823 (2020)
H. Yamazaki, K. Sugino, K. Matsuzu, C. Masaki, J. Akaishi, K. Hames, C. Tomoda, A. Suzuki, T. Uruno, K. Ohkuwa, W. Kitagawa, M. Nagahama, M. Masuda, K. Ito, Rapid disease progression after discontinuation of lenvatinib in thyroid cancer. Medicine 99, e19408 (2020)
K. Sugino, M. Nagahama, W. Kitagawa, K. Ohkuwa, T. Uruno, K. Matsuzu, A. Suzuki, C. Masaki, J. Akaishi, K.Y. Hames, C. Tomoda, Y. Ogimi, K. Ito, Clinical factors related to the efficacy of tyrosine kinase inhibitor therapy in radioactive iodine refractory recurrent differentiated thyroid cancer patients. Endocr. J. 65, 299–306 (2018)
M.E. Cabanillas, M. Schlumberger, B. Jarzab, R.G. Martins, F. Pacini, B. Robinson, J.C. McCaffrey, M.H. Shah, D.L. Bodenner, D. Topliss, C. Andresen, J.P. O’Brien, M. Ren, Y. Funahashi, R. Allison, R. Elisei, K. Newbold, L.F. Licitra, S.I. Sherman, D.W. Ball, A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: a clinical outcomes and biomarker assessment. Cancer 121, 2749–2756 (2015)
M.S. Brose, Y. Panaseykin, B. Konda, C. de la Fouchardiere, B.G.M. Hughes, A.G. Gianoukakis, Y. Joo Park, I. Romanov, M.K. Krzyzanowska, S. Leboulleux, T.A. Binder, C. Dutcus, R. Xie, M.H. Taylor, A randomized study of lenvatinib 18 mg vs 24 mg in patients with radioiodine-refractory differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 107, 776–787 (2020)
E. Song, M. Kim, E.Y. Kim, B.H. Kim, D.Y. Shin, H.C. Kang, B.C. Ahn, W.B. Kim, Y.K. Shong, M.J. Jeon, D.J. Lim, Lenvatinib for radioactive iodine-refractory differentiated thyroid carcinoma and candidate biomarkers associated with survival: a multicenter study in Korea. Thyroid 30, 732–738 (2020)
H.J. Jiang, Y.H. Chang, Y.H. Chen, C.W. Wu, P.W. Wang, P.J. Hsiao, Low dose of lenvatinib treatment for patients of radioiodine-refractory differentiated thyroid carcinoma—a real-world experience. Cancer Manag. Res. 13, 7139–7148 (2021)
T. Ozeki, M. Nagahama, K. Fujita, A. Suzuki, K. Sugino, K. Ito, M. Miura, Influence of CYP3A4/5 and ABC transporter polymorphisms on lenvatinib plasma trough concentrations in Japanese patients with thyroid cancer. Sci. Rep. 9, 5404 (2019)
M. Nagahama, T. Ozeki, A. Suzuki, K. Sugino, T. Niioka, K. Ito, M. Miura, Association of lenvatinib trough plasma concentrations with lenvatinib-induced toxicities in Japanese patients with thyroid cancer. Med Oncol. 36, 39 (2019)
H. Iwamoto, H. Suzuki, S. Shimose, T. Niizeki, M. Nakano, T. Shirono, S. Okamura, Y. Noda, N. Kamachi, T. Nakamura, A. Masuda, T. Sakaue, T. Tanaka, D. Nakano, M. Sakai, T. Yamaguchi, R. Kuromatsu, H. Koga, T. Torimura, Weekends-off lenvatinib for unresectable hepatocellular carcinoma improves therapeutic response and tolerability toward adverse events. Cancers 12, 1010 (2020)
M. Tahara, M. Schlumberger, R. Elisei, M.A. Habra, N. Kiyota, R. Paschke, C.E. Dutcus, T. Hihara, S. McGrath, M. Matijevic, T. Kadowaki, Y. Funahashi, S.I. Sherman, Exploratory analysis of biomarkers associated with clinical outcomes from the study of lenvatinib in differentiated cancer of the thyroid. Eur. J. Cancer 75, 213–221 (2017)
H. Yamazaki, T. Yokose, H. Hayashi, H. Iwasaki, S. Osanai, N. Suganuma, H. Nakayama, K. Masudo, Y. Rino, M. Masuda, Expression of fibroblast growth factor receptor 4 and clinical response to lenvatinib in patients with anaplastic thyroid carcinoma: a pilot study. Eur. J. Clin. Pharm. 76, 703–709 (2020)
Acknowledgements
We thank Enago (https://www.enago.jp/) for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Kanagawa Cancer Center (IRB approval no. 2022-4) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamazaki, H., Iwasaki, H., Masudo, K. et al. Prognostic significance of lung metastasis-related finding in lenvatinib treatment for differentiated thyroid cancer. Endocrine 78, 543–551 (2022). https://doi.org/10.1007/s12020-022-03183-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03183-9