Abstract
Purpose
Bone metastases (BM) affect 10–30% of patients with small intestine neuroendocrine tumors (siNET), but little descriptive data are available regarding their distribution throughout the skeleton or potential risk factors. Aim of the study is to better describe the imaging characteristics, distribution, and risk factors of siNET bone metastases using 18F-FDOPA PET/CT.
Methods
All patients with well-differentiated siNET who underwent an 18F-DOPA PET/CT examination in our institution were retrospectively screened between October 2017 and February 2020. Location, SUVmax and CT density of each BM were collected. Sex, metabolic tumor volume (MTV) excluding bone, and metastatic sites other than bone were studied to determine risk factors of BM.
Results
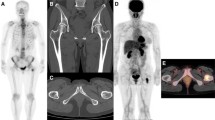
Among the 69 patients included, 11 patients (15.9%) presented BM on 18F-FDOPA (65 metastases). The most frequently involved sites were: thoracic spine, pelvic bones and ribs. About 64% of patients presented multiple BM. On coupled CT scan, 63% of BM were not visible. Using an optimal threshold of 19.2 ml, MTV was an independent predictor of BM (p = 0.004) with a derived sensitivity of 100% [65.0–100.0] and a specificity of 70.9% [57.7–81.2]. Hepatic metastatic involvement was also a significant predictor of BM (p = 0.044).
Conclusion
The development of BM in siNETs appears to be a late event, occurring in patients with a high tumor burden and hepatic involvement. They are often multiple and predominate in the axial skeleton.


Similar content being viewed by others
References
J. Hallet, C.H.L. Law, M. Cukier, R. Saskin, N. Liu, S. Singh, Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 121, 589–597 (2015). https://doi.org/10.1002/cncr.29099
A. Dasari, C. Shen, D. Halperin, B. Zhao, S. Zhou, Y. Xu, T. Shih, J.C. Yao, Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 3, 1335–1342 (2017). https://doi.org/10.1001/jamaoncol.2017.0589
B. Altieri, C. Di Dato, C. Martini, C. Sciammarella, A. Di Sarno, A. Colao, A. Faggiano, Bone metastases in neuroendocrine neoplasms: from pathogenesis to clinical management. Cancers 11, 1332 (2019). https://doi.org/10.3390/cancers11091332
M. Pavel, E. Baudin, A. Couvelard, E. Krenning, K. Öberg, T. Steinmüller, M. Anlauf, B. Wiedenmann, R. Salazar; Barcelona Consensus Conference participants, ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 95, 157–176 (2012). https://doi.org/10.1159/000335597
N. Deleval, L. Pesque, A. Dieudonné, F. Viry, O. Hentic, R. Lebtahi, P. Ruszniewski, L. de Mestier, Prognostic impact of bone metastases detected by 18F-DOPA PET in patients with metastatic midgut neuroendocrine tumors. Eur. Radiol. 31, 4166–4174 (2021). https://doi.org/10.1007/s00330-020-07554-6
M. Scharf, V. Petry, H. Daniel, A. Rinke, T.M. Gress, Bone metastases in patients with neuroendocrine neoplasm: frequency and clinical, therapeutic, and prognostic relevance. Neuroendocrinology 106, 30–37 (2018). https://doi.org/10.1159/000457954
W.G. Meijer, E. Veer, P.L. van der; Jager, E.J. Jagt, B.A. van der; Piers, I.P. Kema, E.G.Ede Vries, P.H.B. Willemse, Bone metastases in carcinoid tumors: clinical features, imaging characteristics, and markers of bone metabolism. J. Nucl. Med. 44, 184–191 (2003)
K. Van Loon, L. Zhang, J. Keiser, C. Carrasco, K. Glass, M.-T. Ramirez, S. Bobiak, E.K. Nakakura, A.P. Venook, M.H. Shah et al. Bone metastases and skeletal-related events from neuroendocrine tumors. Endocr. Connect. 4, 9–17 (2015). https://doi.org/10.1530/EC-14-0119
E.M. Ross, W.C. Roberts, The carcinoid syndrome: comparison of 21 necropsy subjects with carcinoid heart disease to 15 necropsy subjects without carcinoid heart disease. Am. J. Med. 79, 339–354 (1985). https://doi.org/10.1016/0002-9343(85)90313-4
M.F. Bozkurt, I. Virgolini, S. Balogova, M. Beheshti, D. Rubello, C. Decristoforo, V. Ambrosini, A. Kjaer, R. Delgado-Bolton, J. Kunikowska et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 44, 1588–1601 (2017). https://doi.org/10.1007/s00259-017-3728-y
L. de Mestier, C. Lepage, E. Baudin, R. Coriat, F. Courbon, A. Couvelard, C. Do Cao, E. Frampas, S. Gaujoux, R. Gincul et al. Digestive neuroendocrine neoplasms (NEN): French intergroup clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig. Liver Dis. 52, 473–492 (2020). https://doi.org/10.1016/j.dld.2020.02.011
F. Montravers, D. Grahek, K. Kerrou, P. Ruszniewski, V. de Beco, N. Aide, F. Gutman, J.-D. Grangé, J.-P. Lotz, J.-N. Talbot, Can fluorodihydroxyphenylalanine PET replace somatostatin receptor scintigraphy in patients with digestive endocrine tumors? J. Nucl. Med. 47, 1455–1462 (2006)
K.P. Koopmans, O.C. Neels, I.P. Kema, P.H. Elsinga, W.J. Sluiter, K. Vanghillewe, A.H. Brouwers, P.L. Jager, E.G.E. de Vries, Improved staging of patients with carcinoid and islet cell tumors with 18F-Dihydroxy-Phenyl-Alanine and 11C-5-hydroxy-tryptophan positron emission tomography. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 26, 1489–1495 (2008). https://doi.org/10.1200/JCO.2007.15.1126
A. Becherer, M. Szabó, G. Karanikas, P. Wunderbaldinger, P. Angelberger, M. Raderer, A. Kurtaran, R. Dudczak, K. Kletter, Imaging of advanced neuroendocrine tumors with (18)F-FDOPA PET. J. Nucl. Med. Publ. Soc. Nucl. Med. 45, 1161–1167 (2004)
F. Montravers, K. Kerrou, V. Nataf, V. Huchet, J.-P. Lotz, P. Ruszniewski, P. Rougier, F. Duron, P. Bouchard, J.-D. Grangé et al. Impact of fluorodihydroxyphenylalanine-18F positron emission tomography on management of adult patients with documented or occult digestive endocrine tumors. J. Clin. Endocrinol. Metab. 94, 1295–1301 (2009). https://doi.org/10.1210/jc.2008-1349
C. Ansquer, Y. Touchefeu, A. Faivre-Chauvet, C. Leux, M. Le Bras, N. Régenet, V. Fleury, B. Maucherat, H. Senellart, S. Guyetant et al. Head-to-head comparison of 18F-DOPA PET/CT and 68Ga-DOTANOC PET/CT in patients with midgut neuroendocrine tumors. Clin. Nucl. Med. 46, 181–186 (2021). https://doi.org/10.1097/RLU.0000000000003450
A. Piccardo, F. Fiz, G. Bottoni, M. Ugolini, W. Noordzij, P. Trimboli, Head-to-head comparison between 18 F-DOPA PET/CT and 68 Ga-DOTA peptides PET/CT in detecting intestinal neuroendocrine tumours: a systematic review and meta-analysis. Clin. Endocrinol. 95, 595–605 (2021). https://doi.org/10.1111/cen.14527
E. Ouvrard, L.D. Mestier, C. Boursier, B. Lachachi, N. Sahakian, E. Chevalier, N. Mikail, J. Carullo, A. Bando-Delaunay, T. Walter et al. 18F-Fluorodihydroxyphenylalanine PET/CT at the forefront for initial and/or pre-surgical evaluation of small intestine neuroendocrine tumors. J. Nucl. Med. (2022). https://doi.org/10.2967/jnumed.122.263984
D. Morland, P. Jallerat, H. Brixi, G. Cadiot, D. Papathanassiou, S. Deguelte, Performances of 18F-FDOPA PET/CT in the preoperative evaluation of the peritoneal cancer index in small intestine neuroendocrine tumors. Clin. Nucl. Med. 47, 294–298 (2022). https://doi.org/10.1097/RLU.0000000000004057
I.D. Nagtegaal, R.D. Odze, D. Klimstra, V. Paradis, M. Rugge, P. Schirmacher, K.M. Washington, F. Carneiro, I.A. Cree; WHO Classification of Tumours Editorial Board, The 2019 WHO classification of tumours of the digestive system. Histopathology 76, 182–188 (2020). https://doi.org/10.1111/his.13975
B. Kos-Kudła, D. O’Toole, M. Falconi, D. Gross, G. Klöppel, A. Sundin, J. Ramage, K. Oberg, B. Wiedenmann, P. Komminoth et al. ENETS consensus guidelines for the management of bone and lung metastases from neuroendocrine tumors. Neuroendocrinology 91, 341–350 (2010). https://doi.org/10.1159/000287255
E. Garcia-Torralba, F. Spada, K.H.J. Lim, T. Jacobs, J. Barriuso, W. Mansoor, M.G. McNamara, R.A. Hubner, P. Manoharan, N. Fazio et al. Knowns and unknowns of bone metastases in patients with neuroendocrine neoplasms: a systematic review and meta-analysis. Cancer Treat. Rev. 94, 102168 (2021). https://doi.org/10.1016/j.ctrv.2021.102168
D. Morland, L. Jallet, S. Deguelte, G. Cadiot, D. Papathanassiou, Orbital metastasis: a rare but typical location of small intestine neuroendocrine tumor on 18F-FDOPA PET/CT. Clin. Nucl. Med. (2022). https://doi.org/10.1097/RLU.0000000000004137
E. Ziu, V.K. Viswanathan, F.B. Mesfin, Spinal metastasis, (StatPearls Publishing, 2021)
J. Kavecansky, L. Wei, L. Caronia, M.-T. Ramirez, M. Bloomston, M.H. Shah, Bone metastases in well-to-moderately differentiated neuroendocrine tumors: a single institutional review from the Ohio State University Medical Center. Pancreas 44, 198–203 (2015). https://doi.org/10.1097/MPA.0000000000000267
J.M. Zuetenhorst, C.A. Hoefnageli, H. Boot, R.A. Valdés Olmos, B.G. Taal, Evaluation of (111)In-Pentetreotide, (131)I-MIBG and bone scintigraphy in the detection and clinical management of bone metastases in carcinoid disease. Nucl. Med. Commun. 23, 735–741 (2002). https://doi.org/10.1097/00006231-200208000-00006
M. Riihimäki, A. Hemminki, K. Sundquist, J. Sundquist, K. Hemminki, The epidemiology of metastases in neuroendocrine tumors. Int. J. Cancer 139, 2679–2686 (2016). https://doi.org/10.1002/ijc.30400
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by ML and DM. The first draft of the manuscript was written by ML and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This retrospective study was conducted in accordance with the Declaration of Helsinki and was declared on the Health Data Hub (N°F20220506115447) in conformity with the reference methodology MR004 of the “Commission Nationale de l’Informatique et des Libertés”, allowing the computerized management of medical data.
Consent to participate
The participants were informed of the possibility of using the information concerning them and had a right of opposition.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lelièvre, M., Triumbari, E.K.A., Brixi, H. et al. Bone metastases in midgut neuroendocrine tumors: imaging characteristics, distribution, and risk factors. Endocrine 78, 380–386 (2022). https://doi.org/10.1007/s12020-022-03160-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03160-2