Abstract
Background
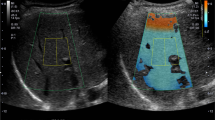
Controlled attenuation parameter (CAP) has been suggested as a new non-invasive measurement performed during transient elastography (TE) to assess liver steatosis. The aim of this study was to evaluate CAP values head to head with ultrasound (US) as reference standard.
Methods
A consecutive cohort of patients attending abdominal US in an outpatient liver unit was included in this study with simultaneous CAP determination using the FibroScan® M probe and fibrosis scored by TE. Patients were subdivided in four groups on the basis of risk factors for Metabolically Associated Fatty Liver Disease (MAFLD).
Results
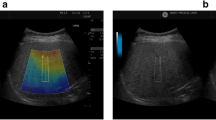
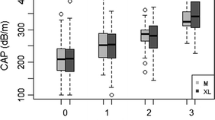
Four hundred thirty-five patients were included in the analysis: 221 (51%) were male; 117 (26.9%) were in control group, 144 (33.1%) in group 2 with inactive HCV or HBV infection and at low-risk for MAFLD, 134 (30.8%) in group 3 at high-risk of MAFLD, 40 (9.2%) in group 4 at high-risk of MAFLD and concomitant inactive HCV or HBV infection. Liver steatosis detected with US evaluation was observed in the 41% of the entire cohort; in particular in the 3.4%, 20.1%, 83.6% and 87.4% of the group 1, 2, 3 and 4, respectively (p < 0.001). In patients at high-risk factor for MAFLD (group 3 and 4), CAP median levels were found statistically different among the severity-grading groups for US steatosis (S0 [n.27], ≥S1 [n.59], ≥S2 + S3 [n.89]), observing higher CAP levels in patients with a higher steatosis grade (≥S2 + S3 327.5 [±40.6] vs ≥S1 277.7 [±45.6] vs S0 245.1 [±47.4]; p < 0.001 for the whole cohort analysis) (p < 0.001 between ≥S2 + S3 and ≥S1) (p < 0.001 between ≥S2 + S3 and S0) (p = 0.004 between ≥S1 and S0). ROC analysis showed that the global performance of the CAP median level ≥ 258 to predict liver steatosis (S0 vs S1–3), was excellent with an Area Under the Curve (AUC) value of 0.87 [CI 95% 0. 835–0.904] with an 84% of sensitivity and a 78% of specificity, and a positive predictive value (PPV) of 73% and negative predictive value (NPV) of 88%. A TE-kPa median value <8.0 was detected in the 100%, 84%, 83.6% and 60% of patients in group 1, 2, 3 and 4, respectively. A TE-kPa median value >13.0 was detected in the 0%, 4.2%, 5.2% and 17.5% of patients in group 1, 2, 3 and 4, respectively.
Conclusions
CAP values are strongly associated with the standard US criteria for different degree of steatosis. Integrating TE up to 5% of patients may be identified at risk for advanced fibrosis.


Similar content being viewed by others
Data availability
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO), EASL-EASD-EASO Clinical Practice Guidelines for the management of nonalcoholic fatty liver disease. J. Hepatol. 64(6), 1388–1402 (2016). https://doi.org/10.1016/j.jhep.2015.11.004
D.E. Kleiner, E.M. Brunt, M. Van Natta et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41(6), 1313–1321 (2005). https://doi.org/10.1002/hep.20701
A. Lonardo, F. Nascimbeni, A. Mantovani, G. Targher, Hypertension, diabetes, atherosclerosis and NASH: cause or consequence. J. Hepatol. 68(2), 335–352 (2018). https://doi.org/10.1016/j.jhep.2017.09.021
S. Bellentani, F. Scaglioni, M. Marino, G. Bedogni, Epidemiology of non-alcoholic fatty liver disease. Dig. Dis. 28(1), 155–161 (2010). https://doi.org/10.1159/000282080
N. Chalasani, Z. Younossi, J.E. Lavine et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67(1), 328–357 (2018). https://doi.org/10.1002/hep.29367
J. Ma, S.J. Hwang, A. Pedley et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J. Hepatol. 66(2), 390–397 (2017). https://doi.org/10.1016/j.jhep.2016.09.022
F. Bril, K. Cusi, Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care 40(3), 419–430 (2017). https://doi.org/10.2337/dc16-1787
K. Lau, R. Lorbeer, R. Haring et al. The association between fatty liver disease and blood pressure in a population-based prospective longitudinal study. J. Hypertens. 28(9), 1829–1835 (2010). https://doi.org/10.1097/HJH.0b013e32833c211b
N. Assy, K. Kaita, D. Mymin, C. Levy, B. Rosser, G. Minuk, Fatty infiltration of liver in hyperlipidemic patients. Dig. Dis. Sci. 45(10), 1929–1934 (2000). https://doi.org/10.1023/a:1005661516165
M. Eslam, P.N. Newsome, S.K. Sarin et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73(1), 202–209 (2020). https://doi.org/10.1016/j.jhep.2020.03.039
F. Bril, C. Ortiz-Lopez, R. Lomonaco et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 35(9), 2139–2146 (2015). https://doi.org/10.1111/liv.12840
V. de Lédinghen, J. Vergniol, J. Foucher, W. Merrouche, B. le Bail, Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 32(6), 911–918 (2012). https://doi.org/10.1111/j.1478-3231.2012.02820.x
European Association for the Study of the Liver, Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel; Chair:; EASL Governing Board representative:; Panel members:. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J. Hepatol. 75(3), 659–689 (2021). https://doi.org/10.1016/j.jhep.2021.05.025
S.M. Grundy, J.I. Cleeman, S.R. Daniels, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement [published correction appears in Circulation. 2005 Oct 25;112(17):e297] [published correction appears in Circulation. 2005 Oct 25;112(17):e298]. Circulation 112(17), 2735–2752 (2005). https://doi.org/10.1161/CIRCULATIONAHA.105.169404
M. Hamaguchi, T. Kojima, Y. Itoh et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 102(12), 2708–2715 (2007). https://doi.org/10.1111/j.1572-0241.2007.01526.x
Q. Li, M. Dhyani, J.R. Grajo, C. Sirlin, A.E. Samir, Current status of imaging in nonalcoholic fatty liver disease. World J. Hepatol. 10(8), 530–542 (2018). https://doi.org/10.4254/wjh.v10.i8.530
T. Karlas, D. Petroff, M. Sasso et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J. Hepatol. 66(5), 1022–1030 (2017). https://doi.org/10.1016/j.jhep.2016.12.022
P.J. Eddowes, M. Sasso, M. Allison et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 156(6), 1717–1730 (2019). https://doi.org/10.1053/j.gastro.2019.01.042
R. Hernaez, M. Lazo, S. Bonekamp et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 54(3), 1082–1090 (2011). https://doi.org/10.1002/hep.24452
Y.N. Zhang, K.J. Fowler, G. Hamilton et al. Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging. Br. J. Radio. 91(1089), 20170959 (2018). https://doi.org/10.1259/bjr.20170959
G. Ferraioli, A. Berzigotti, R.G. Barr et al. Quantification of liver fat content with ultrasound: a WFUMB position paper. Ultrasound Med Biol. 47(10), 2803–2820 (2021). https://doi.org/10.1016/j.ultrasmedbio.2021.06.002
K. Cusi, S. Isaacs, D. Barb et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pr. 28(5), 528–562 (2022). https://doi.org/10.1016/j.eprac.2022.03.010
B.R. Chen, C.Q. Pan, Non-invasive assessment of fibrosis and steatosis in pediatric non-alcoholic fatty liver disease. Clin. Res Hepatol. Gastroenterol. 46(1), 101755 (2022). https://doi.org/10.1016/j.clinre.2021.101755
G. Ferraioli, A. Berzigotti, R.G. Barr et al. Quantification of liver fat content with ultrasound: a WFUMB position paper. Ultrasound Med Biol. 47(10), 2803–2820 (2021). https://doi.org/10.1016/j.ultrasmedbio.2021.06.002
M.S. Siddiqui, R. Vuppalanchi, M.L. Van Natta et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 17(1), 156–163.e2 (2019). https://doi.org/10.1016/j.cgh.2018.04.043
S.B. Ahn, D.W. Jun, B.K. Kang, M. Kim, M. Chang, E. Nam, Optimal cutoff value for assessing changes in intrahepatic fat amount by using the controlled attenuation parameter in a longitudinal setting. Med. (Baltim.) 97(50), e13636 (2018). https://doi.org/10.1097/MD.0000000000013636
V.W. Wong, S. Petta, J.B. Hiriart et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J. Hepatol. 67(3), 577–584 (2017). https://doi.org/10.1016/j.jhep.2017.05.005
V. Rosato, A. Ascione, R. Nevola et al. Factors affecting long-term changes of liver stiffness in direct-acting anti-hepatitis C virus therapy: a multicentre prospective study. J. Viral Hepat. 29(1), 26–34 (2022). https://doi.org/10.1111/jvh.13617
A. Salmi, One-minute liver “digitopsy” for point of care risk stratification of fibrosis in nonalcoholic fatty liver disease. Minerva Gastroenterol. (Torino) 67(2), 209–210 (2021). https://doi.org/10.23736/S2724-5985.20.02700-2
A. Salmi, G. Lanzani, P. Campagnola, L. Frulloni,, Teaching echoscopy for the early diagnosis of ascites in cirrhosis: assessment of an objective structured clinical examination (OSCE). J. Ultrasound 20(2), 123–126 (2017). https://doi.org/10.1007/s40477-017-0250-9
Author contributions
A.S. contributed to the study conception and design, material preparation, investigation, data collection and analysis. The first draft of the manuscript was written by A.S. L.d.F. and C.F. contributed to statistical analyses, material preparation. S.F. and A.G. revised and commented versions of the manuscript. All authors read and approved the final manuscript. The Work submitted for publication is original and has not been published in any language or format and has not been submitted elsewhere for print or electronic publication consideration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Informed consent
Informed consent regarding data collection and anonymous publication of data was signed by all participants as reported in text.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salmi, A., di Filippo, L., Ferrari, C. et al. Ultrasound and FibroScan® Controlled Attenuation Parameter in patients with MAFLD: head to head comparison in assessing liver steatosis. Endocrine 78, 262–269 (2022). https://doi.org/10.1007/s12020-022-03157-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03157-x