Abstract
Purpose
Recent studies claim that immune checkpoint inhibitors are effective in defective mismatch repair (dMMR) cancers. This raises the question of whether similar therapies are effective in PanNETs (pancreatic neuroendocrine tumors); however, in general, assessment of MMR status in PanNETs has been inconsistent in previous studies. MGMT (O6-methylguanine-DNA methyltransferase) is potentially important for guiding temozolomide (TMZ) therapy in glioblastoma. The number of reports on MGMT expression and promoter methylation in PanNETs are limited.
Methods
In this study we assessed the expression of MGMT and MMR proteins MSH2, MSH6, MLH1 and PMS2 in a series of PanNETs by IHC. The methylation status of MGMT and MMR genes in a subset of PanNETs was further assessed by MS-MLPA analysis. Survival curves were constructed using the Kaplan-Meier method, and differences were assessed using the log-rank test. Multivariate Cox proportional hazards regression models were used to determine the prognostic value of the variables.
Results
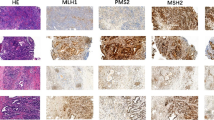
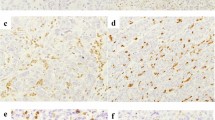
According to evaluation criteria for mismatch repair defects, none of PanNETs shown nuclear staining loss for MSH2, MSH6, MLH1, and PMS2. MGMT low-intensity PanNETs were more commonly found in higher grade, higher Ki67 index and non-functional tumors (P < 0.05). In multivariate analysis, stage III–IV and low-intensity MGMT were shown to be independent risk factors for progression of PanNETs in the entire cohort, non-functioning subgroup and G2 subgroup (P < 0.05 for all). MGMT promoter methylation tended to be higher in the group with low expression of MGMT, However, methylation of MGMT did not statistically correlate with low expression of MGMT (P = 0.153).
Conclusions
In conclusion, our study suggests that decreased expression of MGMT but not MMR is associated with a higher risk of progression of pancreatic neuroendocrine tumors.



Similar content being viewed by others
References
A. Dasari, C. Shen, D. Halperin, B. Zhao, S. Zhou, Y. Xu, T. Shih, J.C. Yao, Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 3(10), 1335–1342 (2017). https://doi.org/10.1001/jamaoncol.2017.0589
C.G. Tran, A.T. Scott, G. Li, S.K. Sherman, P.H. Ear, J.R. Howe, Metastatic pancreatic neuroendocrine tumors have decreased somatostatin expression and increased Akt signaling. Surgery 169(1), 155–161 (2021). https://doi.org/10.1016/j.surg.2020.04.034
G. Perri, L.R. Prakash, M.H.G. Katz, Pancreatic neuroendocrine tumors. Curr. Opin. Gastroenterol. 35(5), 468–477 (2019). https://doi.org/10.1097/mog.0000000000000571
G.B. Mpilla, P.A. Philip, B. El-Rayes, A.S. Azmi, Pancreatic neuroendocrine tumors: Therapeutic challenges and research limitations. World J. Gastroenterol. 26(28), 4036–4054 (2020). https://doi.org/10.3748/wjg.v26.i28.4036
D. Liu, G. Keijzers, L.J. Rasmussen, DNA mismatch repair and its many roles in eukaryotic cells. Mutat. Res. Rev. Mutat. Res. 773, 174–187 (2017). https://doi.org/10.1016/j.mrrev.2017.07.001
D.T. Le, J.N. Durham, K.N. Smith, H. Wang, B.R. Bartlett, L.K. Aulakh, S. Lu, H. Kemberling, C. Wilt, B.S. Luber, F. Wong, N.S. Azad, A.A. Rucki, D. Laheru, R. Donehower, A. Zaheer, G.A. Fisher, T.S. Crocenzi, J.J. Lee, T.F. Greten, A.G. Duffy, K.K. Ciombor, A.D. Eyring, B.H. Lam, A. Joe, S.P. Kang, M. Holdhoff, L. Danilova, L. Cope, C. Meyer, S. Zhou, R.M. Goldberg, D.K. Armstrong, K.M. Bever, A.N. Fader, J. Taube, F. Housseau, D. Spetzler, N. Xiao, D.M. Pardoll, N. Papadopoulos, K.W. Kinzler, J.R. Eshleman, B. Vogelstein, R.A. Anders, L.A. Diaz Jr., Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357(6349), 409–413 (2017). https://doi.org/10.1126/science.aan6733
M.G. House, J.G. Herman, M.Z. Guo, C.M. Hooker, R.D. Schulick, K.D. Lillemoe, J.L. Cameron, R.H. Hruban, A. Maitra, C.J. Yeo, Aberrant hypermethylation of tumor suppressor genes in pancreatic endocrine neoplasms. Ann. Surg. 238(3), 423–431 (2003). https://doi.org/10.1097/01.sla.0000086659.49569.9e. discussion 431–422
M. Mei, D. Deng, T.H. Liu, X.T. Sang, X. Lu, H.D. Xiang, J. Zhou, H. Wu, Y. Yang, J. Chen, C.M. Lu, Y.J. Chen, Clinical implications of microsatellite instability and MLH1 gene inactivation in sporadic insulinomas. J. Clin. Endocrinol. Metab. 94(9), 3448–3457 (2009). https://doi.org/10.1210/jc.2009-0173
T. Arnason, H.L. Sapp, D. Rayson, P.J. Barnes, M. Drewniak, B.A. Nassar, W.Y. Huang, Loss of expression of DNA mismatch repair proteins is rare in pancreatic and small intestinal neuroendocrine tumors. Arch. Pathol. Lab. Med. 135(12), 1539–1544 (2011). https://doi.org/10.5858/arpa.2010-0560-OA
C.N. Arnold, A. Sosnowski, A. Schmitt-Gräff, R. Arnold, H.E. Blum, Analysis of molecular pathways in sporadic neuroendocrine tumors of the gastro-entero-pancreatic system. Int. J. Cancer 120(10), 2157–2164 (2007). https://doi.org/10.1002/ijc.22569
A. Mansouri, L.D. Hachem, S. Mansouri, F. Nassiri, N.J. Laperriere, D. Xia, N.I. Lindeman, P.Y. Wen, A. Chakravarti, M.P. Mehta, M.E. Hegi, R. Stupp, K.D. Aldape, G. Zadeh, MGMT promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro. Oncol. 21(2), 167–178 (2019). https://doi.org/10.1093/neuonc/noy132
M.H. Kulke, J.L. Hornick, C. Frauenhoffer, S. Hooshmand, D.P. Ryan, P.C. Enzinger, J.A. Meyerhardt, J.W. Clark, K. Stuart, C.S. Fuchs, M.S. Redston, O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin. Cancer Res. 15(1), 338–345 (2009). https://doi.org/10.1158/1078-0432.Ccr-08-1476
J.A. Gilbert, L.J. Adhikari, R.V. Lloyd, T.R. Halfdanarson, M.H. Muders, M.M. Ames, Molecular markers for novel therapeutic strategies in pancreatic endocrine tumors. Pancreas 42(3), 411–421 (2013). https://doi.org/10.1097/MPA.0b013e31826cb243
A.O. Chan, S.G. Kim, A. Bedeir, J.P. Issa, S.R. Hamilton, A. Rashid, CpG island methylation in carcinoid and pancreatic endocrine tumors. Oncogene 22(6), 924–934 (2003). https://doi.org/10.1038/sj.onc.1206123
M. Esteller, J.G. Herman, Cancer as an epigenetic disease: DNA methylation and chromatin alterations in human tumours. J. Pathol. 196(1), 1–7 (2002). https://doi.org/10.1002/path.1024
A.O. Nygren, N. Ameziane, H.M. Duarte, R.N. Vijzelaar, Q. Waisfisz, C.J. Hess, J.P. Schouten, A. Errami, M.L.P.A. Methylation-specific, (MS-MLPA): simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acids Res. 33(14), e128 (2005). https://doi.org/10.1093/nar/gni127
R.V. Lloyd, R.Y. Osamura, G. Klöppel, J. Rosai: WHO Classification of Tumours of Endocrine Organs. IARC, Lyon (2017).
M. Intartaglia, R. Sabetta, M. Gargiulo, G. Roncador, F.Z. Marino, R. Franco, Immunohistochemistry for Cancer Stem Cells Detection: Principles and Methods. Methods Mol. Biol. 1692, 195–211 (2018). https://doi.org/10.1007/978-1-4939-7401-6_17
K. Garg, M.M. Leitao Jr., N.D. Kauff, J. Hansen, K. Kosarin, J. Shia, R.A. Soslow, Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am. J. Surg. Pathol. 33(6), 925–933 (2009). https://doi.org/10.1097/PAS.0b013e318197a046
M. Brell, A. Tortosa, E. Verger, J.M. Gil, N. Viñolas, S. Villá, J.J. Acebes, L. Caral, T. Pujol, I. Ferrer, T. Ribalta, F. Graus, Prognostic significance of O6-methylguanine-DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression in anaplastic gliomas. Clin. Cancer Res. 11(14), 5167–5174 (2005). https://doi.org/10.1158/1078-0432.Ccr-05-0230
J. Wen, Y. Wang, M. Yuan, Z. Huang, Q. Zou, Y. Pu, B. Zhao, Z. Cai, Role of mismatch repair in aging. Int J. Biol. Sci. 17(14), 3923–3935 (2021). https://doi.org/10.7150/ijbs.64953
P. Bhattacharjee, T. Sanyal, S. Bhattacharjee, P. Bhattacharjee, Epigenetic alteration of mismatch repair genes in the population chronically exposed to arsenic in West Bengal, India. Environ. Res. 163, 289–296 (2018). https://doi.org/10.1016/j.envres.2018.01.002
U. Herrlinger, T. Tzaridis, F. Mack, J.P. Steinbach, U. Schlegel, M. Sabel, P. Hau, R.D. Kortmann, D. Krex, O. Grauer, R. Goldbrunner, O. Schnell, O. Bähr, M. Uhl, C. Seidel, G. Tabatabai, T. Kowalski, F. Ringel, F. Schmidt-Graf, B. Suchorska, S. Brehmer, A. Weyerbrock, M. Renovanz, L. Bullinger, N. Galldiks, P. Vajkoczy, M. Misch, H. Vatter, M. Stuplich, N. Schäfer, S. Kebir, J. Weller, C. Schaub, W. Stummer, J.C. Tonn, M. Simon, V.C. Keil, M. Nelles, H. Urbach, M. Coenen, W. Wick, M. Weller, R. Fimmers, M. Schmid, E. Hattingen, T. Pietsch, C. Coch, M. Glas, Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet 393(10172), 678–688 (2019). https://doi.org/10.1016/s0140-6736(18)31791-4
B.H. Lok, E.E. Gardner, V.E. Schneeberger, A. Ni, P. Desmeules, N. Rekhtman, E. de Stanchina, B.A. Teicher, N. Riaz, S.N. Powell, J.T. Poirier, C.M. Rudin, PARP Inhibitor activity correlates with SLFN11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin. Cancer Res. 23(2), 523–535 (2017). https://doi.org/10.1158/1078-0432.Ccr-16-1040
B.C. Medeiros, H.E. Kohrt, J. Gotlib, S.E. Coutre, B. Zhang, D.A. Arber, J.L. Zehnder, Tailored temozolomide therapy according to MGMT methylation status for elderly patients with acute myeloid leukemia. Am. J. Hematol. 87(1), 45–50 (2012). https://doi.org/10.1002/ajh.22191
C. Halevy, B.C. Whitelaw, How effective is temozolomide for treating pituitary tumours and when should it be used? Pituitary 20(2), 261–266 (2017). https://doi.org/10.1007/s11102-016-0745-y
R. Tuominen, R. Jewell, J.J. van den Oord, P. Wolter, U. Stierner, C. Lindholm, C. Hertzman Johansson, D. Lindén, H. Johansson, M. Frostvik Stolt, C. Walker, H. Snowden, J. Newton-Bishop, J. Hansson, S. Egyházi Brage, MGMT promoter methylation is associated with temozolomide response and prolonged progression-free survival in disseminated cutaneous melanoma. Int J. Cancer 136(12), 2844–2853 (2015). https://doi.org/10.1002/ijc.29332
F. Morano, S. Corallo, M. Niger, L. Barault, M. Milione, R. Berenato, R. Moretto, G. Randon, M. Antista, A. Belfiore, A. Raimondi, F. Nichetti, A. Martinetti, L. Battaglia, F. Perrone, G. Pruneri, A. Falcone, M. Di Bartolomeo, F. de Braud, F. Di Nicolantonio, C. Cremolini, F. Pietrantonio, Temozolomide and irinotecan (TEMIRI regimen) as salvage treatment of irinotecan-sensitive advanced colorectal cancer patients bearing MGMT methylation. Ann. Oncol. 29(8), 1800–1806 (2018). https://doi.org/10.1093/annonc/mdy197
J. Cros, O. Hentic, V. Rebours, M. Zappa, N. Gille, N. Theou-Anton, D. Vernerey, F. Maire, P. Lévy, P. Bedossa, V. Paradis, P. Hammel, P. Ruszniewski, A. Couvelard, MGMT expression predicts response to temozolomide in pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 23(8), 625–633 (2016). https://doi.org/10.1530/erc-16-0117
R. Della Monica, M. Cuomo, R. Visconti, A. di Mauro, M. Buonaiuto, D. Costabile, G. De Riso, T. Di Risi, E. Guadagno, R. Tafuto, S. Lamia, A. Ottaiano, P. Cappabianca, M.L. Del Basso de Caro, F. Tatangelo, J. Hench, S. Frank, S. Tafuto, L. Chiariotti, Evaluation of MGMT gene methylation in neuroendocrine neoplasms. Oncol. Res. 28(9), 837–845 (2022). https://doi.org/10.3727/096504021x16214197880808
Acknowledgements
We thank Yiwei Yang of Xiamen Zeesan Biotechnology Co., Ltd. for his technical assistance with the MS-MLPA analysis.
Funding
This work was supported by the CAMS Science and Technology Innovation Program Fund for Medical Sciences and Health (Grant number [2016-I2M-1-001]), the National Scientific Data Sharing Platform for Population and Health (Grant number [NCMI–YF01N–201906]), the National Natural Science Foundation of China (C.N.) (Grant numbers [81672648], [81472326], [81341070], and [81400664]), and special funds from the Molecular Pathology Center and the Central Public Welfare Institutions of CAMS (Grant numbers [2016ZX310176-4] and [2017PT31008]). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
S.Y., J.C. and Z.L. contributed to the study conception and design. Material preparation, data collection and analysis were performed by S.mo., C.J., and H.S. Experimental procedure (IHC and MS-MLPA) was performed by X.B., Y.Z., J.P., and Y.Z. X.C. and X.M. reviewed the H&E slide and evaluated the IHC results. The first draft of the manuscript was written by X.B. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Peking Union Medical College Hospital (No. [S-K523]).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The manuscript does not contain any personal data of any kind (including any personal details, images or videos), Ethics Committee has confirmed that no consent for publication is required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ban, X., Mo, S., Lu, Z. et al. Expression and methylation status of MMR and MGMT in well-differentiated pancreatic neuroendocrine tumors and potential clinical applications. Endocrine 77, 538–545 (2022). https://doi.org/10.1007/s12020-022-03102-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03102-y