Abstract
Purpose
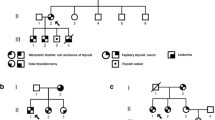
Papillary thyroid carcinoma (PTC) is the most common type of thyroid carcinoma and its incidence has greatly increased in the last 30 years. Ubiquitin-specific protease 13 (USP13) is a class of deubiquitinating enzymes (DUBs) and plays an important role in cellular functions such as cell cycle regulation, DNA damage repair, and different cell signaling pathways. Studies regarding the role of USP13 in cancer development and progression are divergent and there are no previous data regarding the role of USP13 gene in PTCs. In this study, we investigated the genetic cause of PTC diagnosed in multiple members of a Brazilian family.
Methods
Whole exome sequencing (WES) was performed to identify the genetic cause of PTC. Cycloheximide chase assay and clonogenic assay were performed to study USP13 stability and function in vitro.
Results
WES analysis identified a heterozygous missense variant c.1483G > A (p.V495M) in the USP13 gene that fully segregates with the disease. In silico modeling suggests that this variant may cause protein structural perturbations. USP13 overexpression increased the potential of a single cell to form colonies. The USP13 c.1483G > A variant enhanced the effects seen in USP13 overexpression and preserved protein stability for longer hours compared to the non-mutated USP13 protein.
Conclusion
Our study suggests that USP13 overexpression may play a role in tumorigenesis of PTCs; and that the USP13 p.V495M (c.1483G > A) variant enhances USP13 estability.




Similar content being viewed by others
References
A. Rangel-Pozzo, L. Sisdelli, M.I.V. Cordioli, F. Vaisman, P. Caria, S. Mai, et al. Genetic landscape of papillary thyroid carcinoma and nuclear architecture: an overview comparing pediatric and adult populations. Cancers. 12(11), 3146.
E. Macerola, A.M. Poma, P. Vignali, A. Basolo, C. Ugolini, L. Torregrossa, et al. Molecular genetics of follicular-derived thyroid cancer. Cancers. 2021;13(5), 1139.
J. Krajewska, A. Kukulska, M. Oczko-Wojciechowska, A. Kotecka-Blicharz, K. Drosik-Rutowicz, M. Haras-Gil et al. Early diagnosis of low-risk papillary thyroid cancer results rather in overtreatment than a better survival. Front Endocrinol 11, 571421 (2020)
M.I. Abdullah, S.M. Junit, K.L. Ng, J.J. Jayapalan, B. Karikalan, O.H. Hashim, Papillary thyroid cancer: genetic alterations and molecular biomarker investigations. Int J Med Sci 16(3), 450–460 (2019)
L. Raposo, S. Morais, M.J. Oliveira, A.P. Marques, M. Jose Bento, N. Lunet, Trends in thyroid cancer incidence and mortality in Portugal. Eur J Cancer Prev 26(2), 135–143 (2017)
Y. Fang, X. Ma, J. Zeng, Y. Jin, Y. Hu, J. Wang et al. The profile of genetic mutations in papillary thyroid cancer detected by whole exome sequencing. Cell Physiol Biochem 50(1), 169–178 (2018)
N. Pozdeyev, L.M. Gay, E.S. Sokol, R. Hartmaier, K.E. Deaver, S. Davis et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res 24(13), 3059–3068 (2018)
Nylen C., Mechera R., Marechal-Ross I., Tsang V., Chou A., Gill A.J., et al. Molecular markers guiding thyroid cancer management. Cancers. 2020;12(8), 2164.
C.A. Ghossein, S. Dogan, N. Farhat, I. Landa, B. Xu,, Expanding the spectrum of thyroid carcinoma with somatic DICER1 mutation: a survey of 829 thyroid carcinomas using MSK-IMPACT next-generation sequencing platform.Virchows Arch. 480(2), 293–302 (2021).
T.T. Nieminen, C.J. Walker, A. Olkinuora, L.K. Genutis, M. O’Malley, P.E. Wakely et al. Thyroid carcinomas that occur in familial adenomatous polyposis patients recurrently harbor somatic variants in APC, BRAF, and KTM2D. Thyroid. 30(3), 380–388 (2020)
Zhao Y., Yu T., Sun J., Wang F., Cheng C., He S., et al. Germ-line mutations in WDR77 predispose to familial papillary thyroid cancer. Proc Natl Acad. Sci. USA. 2021;118(31), e2026327118.
Cruz L., Soares P., Correia M. Ubiquitin-specific proteases: players in cancer cellular processes. Pharmaceuticals. 2021;14(9), 848.
E.L. Morgan, M.R. Patterson, D. Barba-Moreno, J.A. Scarth, A. Wilson, A. Macdonald, The deubiquitinase (DUB) USP13 promotes Mcl-1 stabilisation in cervical cancer. Oncogene. 40(11), 2112–2129 (2021)
X. Fang, W. Zhou, Q. Wu, Z. Huang, Y. Shi, K. Yang et al. Deubiquitinase USP13 maintains glioblastoma stem cells by antagonizing FBXL14-mediated Myc ubiquitination. J Exp Med 214(1), 245–267 (2017)
X. Man, C. Piao, X. Lin, C. Kong, X. Cui, Y. Jiang, USP13 functions as a tumor suppressor by blocking the NF-kB-mediated PTEN downregulation in human bladder cancer. J Exp Clin Cancer Res 38(1), 259 (2019)
Z. Qu, R. Zhang, M. Su, W. Liu, USP13 serves as a tumor suppressor via the PTEN/AKT pathway in oral squamous cell carcinoma. Cancer Manag Res 11, 9175–9183 (2019)
J. Zhang, P. Zhang, Y. Wei, H.L. Piao, W. Wang, S. Maddika et al. Deubiquitylation and stabilization of PTEN by USP13. Nat Cell Biol 15(12), 1486–1494 (2013)
E.D. Accordi, P. Xekouki, B. Azevedo, R.B. de Alexandre, C. Frasson, S.M. Gantzel et al. Familiar papillary thyroid carcinoma in a large brazilian family is not associated with succinate dehydrogenase defects. Eur Thyroid J. 5(2), 94–99 (2016)
L.F. Azevedo, G.D. Pecharki, J.A. Brancher, C.A. Cordeiro Jr., K.G. Medeiros, A.A. Antunes et al. Analysis of the association between lactotransferrin (LTF) gene polymorphism and dental caries. J Appl Oral Sci 18(2), 166–170 (2010)
L. Drougat, N. Settas, C.L. Ronchi, K. Bathon, D. Calebiro, A.G. Maria et al. Genomic and sequence variants of protein kinase A regulatory subunit type 1beta (PRKAR1B) in patients with adrenocortical disease and Cushing syndrome. Genet Med 23(1), 174–182 (2021)
Espiard S., Knape M.J., Bathon K., Assie G., Rizk-Rabin M., Faillot S., et al. Activating PRKACB somatic mutation in cortisol-producing adenomas. JCI Insight. 2018;3(8), e98296.
M. Mistri, P.M. Tamhankar, F. Sheth, D. Sanghavi, P. Kondurkar, S. Patil et al. Identification of novel mutations in HEXA gene in children affected with Tay Sachs disease from India. PLoS One 7(6), e39122 (2012)
A. Roy, J. Yang, Y. Zhang, COFACTOR: an accurate comparative algorithm for structure-based protein function annotation. Nucleic Acids Res 40(Web Server issue), W471–W477 (2012)
Y. Zhang, I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9, 40 (2008)
S. Xiang, J. Fang, S. Wang, B. Deng, L. Zhu, MicroRNA135b regulates the stability of PTEN and promotes glycolysis by targeting USP13 in human colorectal cancers. Oncol Rep 33(3), 1342–1348 (2015)
Y. Yin, W.H. Shen, PTEN: a new guardian of the genome. Oncogene. 27(41), 5443–5453 (2008)
D. Hanahan, R.A. Weinberg, Hallmarks of cancer: the next generation. Cell. 144(5), 646–674 (2011)
Buchanan B.W., Lloyd M.E., Engle S.M., Rubenstein E.M. Cycloheximide chase analysis of protein degradation in saccharomyces cerevisiae. J Vis Exp 2016 (110), 53975.
Y.J. Lee, Y.J. Cho, Y.J. Heo, E.J. Chung, Y.H. Choi, J.I. Kim et al. Thyroid nodules in childhood-onset Hashimoto’s thyroiditis: Frequency, risk factors, follow-up course and genetic alterations of thyroid cancer. Clin Endocrinol 95(4), 638–648 (2021)
M.J. Young, K.C. Hsu, T.E. Lin, W.C. Chang, J.J. Hung, The role of ubiquitin-specific peptidases in cancer progression. J Biomed Sci 26(1), 42 (2019)
T. Qi, X. Rong, Q. Feng, H. Sun, H. Cao, Y. Yang et al. Somatic mutation profiling of papillary thyroid carcinomas by whole-exome sequencing and its relationship with clinical characteristics. Int J Med Sci 18(12), 2532–2544 (2021)
Siraj S., Masoodi T., Siraj A.K., Azam S., Qadri Z., Parvathareddy S.K., et al. APOBEC SBS13 mutational signature-a novel predictor of radioactive iodine refractory papillary thyroid carcinoma. Cancers. 2022;14(6), 1584.
S. Zhang, M. Zhang, Y. Jing, X. Yin, P. Ma, Z. Zhang et al. Deubiquitinase USP13 dictates MCL1 stability and sensitivity to BH3 mimetic inhibitors. Nat Commun 9(1), 215 (2018)
S. Gao, T. Chen, L. Li, X. Liu, Y. Liu, J. Zhao et al. Hypoxia-inducible ubiquitin specific peptidase 13 contributes to tumor growth and metastasis via enhancing the toll-like Receptor 4/Myeloid differentiation primary response gene 88/Nuclear Factor-kappaB pathway in hepatocellular carcinoma. Front. Cell Dev. Biol. 8, 587389 (2020)
W. Kim, F. Zhao, H. Gao, S. Qin, J. Hou, M. Deng et al. USP13 regulates the replication stress response by deubiquitinating TopBP1. DNA Repair 100, 103063 (2021)
M. Esposito, H.B. Akman, P. Giron, M.A. Ceregido, R. Schepers, L.C. Ramos Paez et al. USP13 controls the stability of Aurora B impacting progression through the cell cycle. Oncogene. 39(37), 6009–6023 (2020)
Funding
This work was funded by the NIH Intramural Grant Z01-HD008920-01 of the Eunice Kennedy Shriver National Institute for Child Health & Human Development (NICHD), Division of Intramural Research (DIR) to Dr. Constantine A. Stratakis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Stratakis holds patents on the function of the PRKAR1A, PDE11A, and GPR101 genes and related issues; his laboratory has also received research funding on GPR101 and its involvement in acromegaly and/or gigantism, abnormal growth hormone secretion and its treatment by Pfizer, Inc.; Dr. Faucz holds patent on the GPR101 gene and/or its function; The other authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Andrea Gutierrez Maria, Bruna Azevedo
Supplementary Information
Rights and permissions
About this article
Cite this article
Maria, A.G., Azevedo, B., Settas, N. et al. USP13 genetics and expression in a family with thyroid cancer. Endocrine 77, 281–290 (2022). https://doi.org/10.1007/s12020-022-03068-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03068-x